Discussion
The IPNV infection induces the synthesis of genes involved in
nonspecific immune response (Robertsen, 2008). Although, the relationship
between the response level and the virus strain virulence is not clear. In the
present study, we evaluated two IPNV serotypes to know if the infectivity level
of the strains affects the signaling pathway of type I interferon alpha
(IFN-I(α)) in RTG-2 cells. The experiment which evaluate the viral replication,
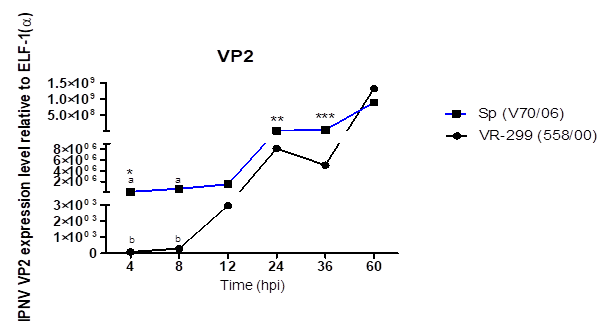
indicates higher virulence of the Sp virus (Wolf, 1988; Dobos, 1995), which
involved the Sp strains in cases of high mortality rates or severe clinical
pictures (Santi et al., 2004; Santi et al., 2005). Sano et al. (1992), related
to the IPNV virulence with segment A, although recently was associated to the
residues 217 and 221 of VP2 protein (Santi et al., 2004). Highly virulent
isolates possess residues Thr217 and Ala221; moderate to low-virulence strains
have Pro217 and Ala221; and strains containing Thr221 are almost avirulent,
irrespective to the residue at position 217 (Song et al., 2005). Based on the above, strains used in this
study corresponding to moderate virulence (Sp serotype) and avirulent (VR-299).
Although, Smail et al. (2006) did not find mortality differences to compare a
high virulent strain with a moderate virulence strain, suggesting that others
factors associated to the strain or immune response possibly affect the
infection findings (Ortega et al., 2011). In our study, we might hypothesize
that higher viral replication before 24 hpi are associated with a greater
penetration for serotype Sp virus, linked to the amino acids residue sequences
of the hypervariable region of VP2 binding protein. In addition, this region
might interact with cell receptors in a different way (Dobos 1995; Kuznar et
al., 1995; Granzow et al., 1997).
Earlier work demonstrated that peptide derived of Vp2 maturation of
infectious bursal disease virus (IBDV) participates in the virus-cell entry
suggesting that peptide 46 (pep 46) has a domain rich in proline (positions
458, 465, 469) that disrupt cell membrane and induces pores (Galloux et al.,
2007). However, in our work we did not find differences between strains in VP2
for peptide 46 (results not shown), therefore, both viruses should behave
similarly, so this peptide 46 is not implicated in viral replication difference between both
serotypes. Therefore, viral replication differences might to be associated with
other structural and functional aspects that have been implicated to IBDV replication
(Da Costa et al., 2002).
Some proteases like IPNV-Vp4 protein have been considered as virulence
factors; however, this finding is not been shown yet (Skjesol et al., 2009);
additionally, the possible involvement of VP5 protein as a virulence factor has
been discarded.
We
demonstrated that, apparently others viral and cellular factors have influenced
that serotype Sp virus show a higher replication. Interestingly the serotype
VR-299 virus reached replication values higher to the Sp at 60 hpi. Studies by Kuznar et al. (1995) showed that
at 10 hpi, viral RNA is detected and at 14 hpi mature particles were detected
also, so this situation might be associated with a random value of viral
multiplicity because at this time-points, more than two replication cycles have
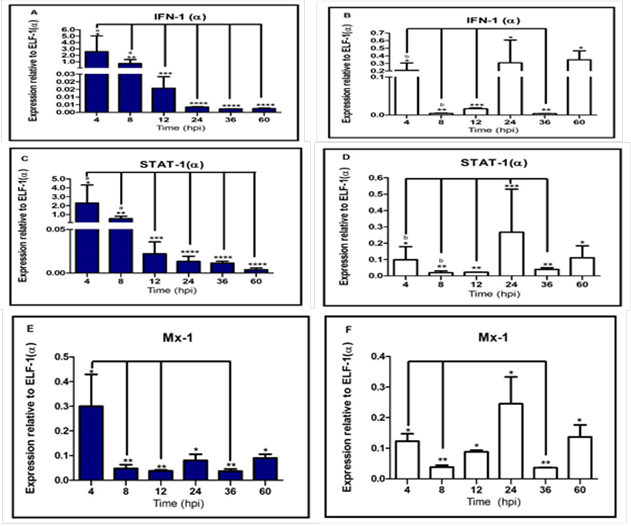
elapsed (Espinoza et al., 2000). IPNV infection induced the expression of
IFN-I(α) that module an antiviral response stimulating the other gene effectors
(Saint-Jean and Pérez-Prieto, 2007). Our results indicate that replication
increase in both serotypes (Sp serotype virus higher than VR-299) was
associated a decreased of IFN-I mRNA, suggesting that, inhibition of alpha
interferon signaling is required for viral replication in early stages of
infection. This finding is consistent with observations from (Skjesol et al.,
2009) that described the ability of IPNV to reduce the interferon immune
response; however, contrary to the hypothesized; the IFN-I(α) expression was
higher in infected cells with serotype Sp virus, suggesting a lineal positive
correlation between strain virulence and alpha interferon immune response.
Although a high expression of
this cytokine might produce a negative effect in cells implicated in high
mortality caused by the cell production of proteases and other proteins that
contribute to cell damage (Hay & Kannourakis, 2002). In contrast to the outcomes
in infected cells with VR-299 serotype virus (avirulent strain), the cellular
response was attenuated. Additionally,
to this study, earlier work demonstrated that IPNV inhibits the mechanism of
interferon signaling (Robertsen, 2008). In relation to this, we showed an
indirect relationship between VP2-IPNV expression and mRNA STAT-1(α)
expression, associated with a decrease of interferon transcript simultaneously,
explained by STAT-1(α) is a ISGs. However, our research also showed that higher
virulence strain is not associated with a greater effect for blocking of alpha
interferon signaling. This outcome can be explained by STAT-1(α) expression was
higher at 8 hpi in infected cells with serotype Sp virus. Additionally, this
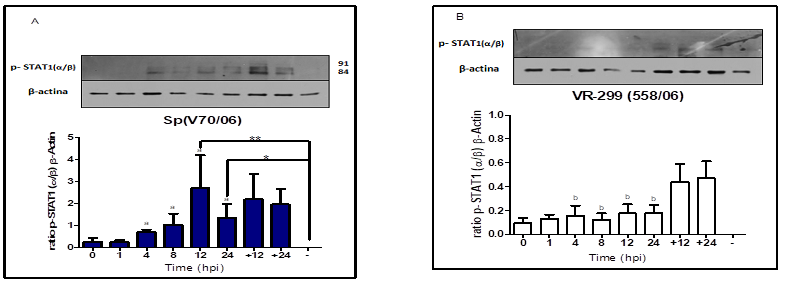
latter finding is supported by higher STAT1(α/β) phosphorylation level induced
by serotype Sp virus compared to serotype VR-299 virus between 4 and 24 hpi
(phosphorylation peak at 12 hpi).
Additionally, we demonstrated that Mx-1 protein inhibit IPNV replication
(Larsen et al., 2004; Jørgensen et al., 2007). However, Mx-1 transcript was
down-regulated simultaneously, the virus replication increased. Possibly, because RTG-2 cells were not priory
stimulated with IFN-I or Poly: IC (Skjesol et al., 2009). Interestingly, the Mx-1 transcript level
between both strains was similar to other study (Ortega et al., 2011), which
reported virulence strain does not affect the Mx expression; we suggest that
both strains has a similar antiviral sensitivity against the interferon
response and ISGs such as Mx protein. A
Mx protein positive effect against infection IPNV possibly is mediated by other
factors such as isoform and amount of protein, cells type, infection
temperature among others (Arguedas et al. 2015). The Y-701 STAT1(α/β)
phosphorylation mediates rapid and robust activity and expression specific
transcriptional of genes for the activating of cytokines and cell factors
growth (Decker et al., 2002; Skjesol et al., 2010). Our results showed that
STAT1(α/β) -pY701 level was significantly increased in infected cells serotype
Sp virus, determined by a significant increase of IFN-I(α) expression at an
early point after infection (4 and 8 hpi) compared to values observed in
infected cell serotype VR-299 virus. This suggest that, 701 STAT1(α/β) tyrosine
was activated by interferon type I(α) in trout, but not by intracellular type
(iIFN-Ib) that was reported for the first time in vertebrates (rainbow trout),
not showing biological activity on STAT1 and STAT2 phosphorylation (Chang et
al., 2013). The alpha interferon
response against IPNV infection induced 701-STAT1(α/β) phosphorylation with
both serotypes. Fascinatingly, this observation illustrate that IPNV does not
inhibit the phosphorylation of 701-tyrosine-STAT1(α/β) stimulated by IFN-I(α),
contrary to other RNA viruses (Horvath, 2004; Randall & Goodbourn, 2008).
Nevertheless, the level Y701-STAT1(α/β) activation is directly correlated with
the strains IPNV virulence. Activation
of STAT1(α/β) in mammals contributes to the maximum transcriptional activation
and apoptosis (Sironi & Ouchi, 2004; Thomas et al., 2004; Townsend et al.,
2004).
Apoptosis previously documented
by IPNV infected fish both in vitro and in vivo independently strain virulence
(Hong et al., 1999; Espinoza et al., 2005; Ortega et al., 2014). Hence, our
results of higher STAT1(α/β) activation found in infected cell with serotype Sp
virus indicates apparently that apoptosis mechanism was required for rapid
elimination of infected cell, such as high cellular response to the strain virulence.
Additionally, the STAT-1(α) and IFN-I(α) expression were down-regulated, that
might be associated to a lower quantity of live cells. Contrary, the
up-regulation gene expression found in infected cell with serotype VR-299 virus
at 24 and 60 hpi which could be a consequence of a higher cell’s ability to
establish an antiviral effect. However, information associated with STAT1(α/β)
phosphorylation after IPNV infection in teleost is limited and the apoptosis
depend completely on the Y-701STAT1 (α/β) phosphorylation and it is an
interesting question that needs to be addressed in future studies. Our data
indicates a negative effect by IPNV on signaling alpha interferon and ISGs
independently to the strain virulence. IPNV does not block the phosphorylation
701STAT1 (α/β) tyrosine contrary to other RNA viruses. Further studies are
clearly needed in order to identify others molecular mechanisms how IPNV
inhibits signaling pathway alpha interferon.
Acknowledgements.
This document was supported by the research project No. 99736 (CONACYT)
and would not have been possible without the allocation of the scholarship
agreement: CONACYT-IICA, Registration No. 283454. We are thankful for FONDAP
15110027: Interdisciplinary Center for Aquaculture Research (INCAR).
References
Arguedas Cortés, D.,
Romero Zuñiga, A. P., Enriquez Sais, R., Martínez Castañeda, J. S., &
Ortega Santana, C. (2015). Effect of temperature on the expression of IFN-1 (α), STAT-1
and Mx-1 genes in Oncorhynchus mykiss
(Salmoniformes: Salmonidae) exposed with the virus of the infectious pancreatic
necrosis (IPNV). Revista de Biología Tropical, 63(2),
559-569.
Chang, M. X., Zou, J., Nie, P., Huang, B., Yu, Z.,
Collet, B., & Secombes, C. J. (2013). Intracellular interferons in fish: a unique
means to combat viral infection. PLoS pathogens, 9(11),
e1003736. doi: 10.1371/journal.ppat.1003736
Collet, B., Ganne, G., Bird, S., & Collins, C. M. (2009).
Isolation and expression profile of a gene encoding for the Signal Transducer
and Activator of Transcription STAT2 in Atlantic salmon (Salmo salar). Developmental & Comparative Immunology, 33(7),
821-829. doi: 10.1016/j.dci.2009.01.007
Collet, B., Munro, E. S., Gahlawat, S., Acosta, F., Garcia,
J., Roemelt, C., ... & Ellis, A. E. (2007). Infectious pancreatic necrosis
virus suppresses type I interferon signalling in rainbow trout gonad cell line
but not in Atlantic salmon macrophages. Fish & Shellfish Immunology, 22(1-2),
44-56. doi: 10.1016/j.fsi.2006.03.011
Da Costa, B., Chevalier, C., Henry, C., Huet, J. C., Petit,
S., Lepault, J., ... & Delmas, B. (2002). The capsid of infectious bursal
disease virus contains several small peptides arising from the maturation
process of pVP2. Journal of virology, 76(5), 2393-2402. doi: 10.1128/jvi.76.5.2393-2402.2002
Darnell, J. E., Kerr, I. M., & Stark, G. R. (1994).
Jak-STAT pathways and transcriptional activation in response to IFNs and other
extracellular signaling proteins. Science, 264(5164),
1415-1421. doi: 10.1126/science.8197455
De Kinkelin, P., & Dorson, M. (1973). Interferon
production in rainbow trout (Salmo
gairdneri) experimentally infected with Egtved virus. Journal of
General Virology, 19(1), 125-127. doi: 10.1099/0022-1317-19-1-125
Decker, T., Stockinger, S., Karaghiosoff, M., Müller, M.,
& Kovarik, P. (2002). IFNs and STATs in innate immunity to
microorganisms. The Journal of clinical investigation, 109(10),
1271-1277. doi: 10.1172/JCI15770
Dobos, P. (1995). The molecular biology of infectious
pancreatic necrosis virus (IPNV). Annual Review of Fish Diseases, 5,
25-54. doi.org/10.1016/0959-8030(95)00003-8
Eaton, W. D. (1990). Anti-viral activity in four species of
salmonids following exposure to poly inosinic: cytidylic acid. Diseases
of Aquatic Organisms, 9(3), 193-198. doi: 10.3354/dao009193
Espinoza, J. C., Cortés-Gutierrez, M., & Kuznar, J.
(2005). Necrosis of infectious pancreatic necrosis virus (IPNV) infected cells
rarely is preceded by apoptosis. Virus
research, 109(2), 133-138. doi:
https://doi.org/10.1016/j.virusres.2004.10.014
Espinoza, J.C., Hjalmarsson, A.,
Everitt, E., & Kuznar, J. (2000). Temporal and subcellular localization of
infectious pancreatic necrosis virus structural proteins. Archives of Virology 145(4): 739-748. doi:
https://doi.org/10.1007/s007050050667
Galloux, M., Libersou, S., Morellet, N., Bouaziz, S., Da
Costa, B., Ouldali, M., ... & Delmas, B. (2007). Infectious bursal disease
virus, a non-enveloped virus, possesses a capsid-associated peptide that
deforms and perforates biological membranes. Journal of Biological Chemistry, 282(28), 20774-20784. doi: 10.1074/jbc.M701048200
García, I., Galiana, A., Falcó, A., Estepa, A., & Perez,
L. (2011). Characterization of an infectious pancreatic necrosis (IPN) virus
carrier cell culture with resistance to superinfection with heterologous
viruses. Veterinary microbiology, 149(1-2),
48-55. doi: 10.1016/j.vetmic.2010.10.017.
Garcin, D., Latorre, P., & Kolakofsky, D. (1999). Sendai
virus C proteins counteract the interferon-mediated induction of an antiviral
state. Journal of Virology, 73(8),
6559-6565.
Gotoh, B., Takeuchi, K., Komatsu, T., Yokoo, J., Kimura, Y.,
Kurotani, A., ... & Nagai, Y. (1999). Knockout of the Sendai virus C gene
eliminates the viral ability to prevent the interferon‐α/β‐mediated responses. FEBS letters, 459(2), 205-210. doi: 10.1016/s0014-5793(99)01241-7
Granzow, H., Weiland, F., Fichtner, D., & Enzmann, P. J.
(1997). Studies of the ultrastructure and morphogenesis of fish pathogenic
viruses grown in cell culture. Journal
of Fish Diseases, 20(1), 1-10. doi:
https://doi.org/10.1046/j.1365-2761.1997.00267.x
Harcourt, B. H., Sanchez, A., & Offermann, M. K. (1999).
Ebola virus selectively inhibits responses to interferons, but not to
interleukin-1β, in endothelial cells. Journal
of virology, 73(4), 3491-3496.
Hay, S., & Kannourakis, G. (2002). A time to kill: viral
manipulation of the cell death program. Journal of General Virology, 83(7), 1547-1564. doi: 10.1099/0022-1317-83-7-1547
Heim, M. H., Moradpour, D., & Blum, H. E. (1999).
Expression of hepatitis C virus proteins inhibits signal transduction through
the Jak-STAT pathway. Journal
of Virology, 73(10), 8469-8475.
Hoeve, J., de Jesus Ibarra-Sanchez, M., Fu, Y., Zhu, W.,
Tremblay, M., David, M., & Shuai, K. (2002). Identification of a nuclear
Stat1 protein tyrosine phosphatase. Molecular
and cellular biology, 22(16), 5662-5668.
doi:
10.1128/mcb.22.16.5662-5668.2002
Hong, J. R., Hsu, Y. L., & Wu, J. L. (1999). Infectious
pancreatic necrosis virus induces apoptosis due to down-regulation of survival
factor MCL-1 protein expression in a fish cell line. Virus research, 63(1-2),
75-83. doi: 10.1016/s0168-1702(99)00060-x
Horvath, C. M. (2004). Weapons of STAT destruction. European
journal of biochemistry, 271(23‐24), 4621-4628. doi:
https://doi.org/10.1111/j.1432-1033.2004.04425.x
International Office of Epizootics. Aquatic Animal Health
Standards Commission. (2006). Manual of diagnostic tests for aquatic
animals. Office International des Epizooties.
Jensen, I., & Robertsen, B. (2002). Effect of
double-stranded RNA and interferon on the antiviral activity of Atlantic salmon
cells against infectious salmon anemia virus and infectious pancreatic necrosis
virus. Fish & shellfish immunology, 13(3), 221-241.
doi: https://doi.org/10.1006/fsim.2001.0397
Jørgensen, J. B., Johansen, A., Hegseth, M. N., Zou, J.,
Robertsen, B., Collet, B., & Secombes, C. J. (2007). A recombinant CHSE-214
cell line expressing an Mx1 promoter–reporter system responds to both
interferons type I and type II from salmonids and represents a versatile tool
to study the IFN-system in teleost fish. Fish & shellfish
immunology, 23(6), 1294-1303. doi: 10.1016/j.fsi.2007.07.008
Komatsu, T., Takeuchi, K., Yokoo, J., Tanaka, Y., & Gotoh,
B. (2000). Sendai virus blocks alpha interferon signaling to signal transducers
and activators of transcription. Journal of virology, 74(5),
2477-2480. doi: 10.1128/JVI.74.5.2477-2480.2000
Kotenko, S. V., Gallagher, G., Baurin, V. V., Lewis-Antes,
A., Shen, M., Shah, N. K., ... & Donnelly, R. P. (2003). IFN-λs mediate
antiviral protection through a distinct class II cytokine receptor
complex. Nature immunology, 4(1), 69. doi: 10.1038/ni875
Kuznar, J., Soler, M., Farias, G. I. L. D. A., &
Espinoza, J. C. (1995). Attachment and entry of infectious pancreatic necrosis
virus (IPNV) into CHSE-214 cells. Archives of virology, 140(10),
1833-1840. doi: 10.1007/bf01384345
Larsen, R., Røkenes, T. P., & Robertsen, B. (2004).
Inhibition of infectious pancreatic necrosis virus replication by Atlantic
salmon Mx1 protein. Journal of virology, 78(15),
7938-7944. doi: 10.1128/JVI.78.15.7938-7944.2004
Leu, J. H., Yan, S. J., Lee, T. F., Chou, C. M., Chen, S. T.,
Hwang, P. P., ... & Huang, C. J. (2000). Complete genomic organization and
promoter analysis of the round-spotted pufferfish JAK 1, JAK 2, JAK 3, and TYK
2 genes. DNA and cell biology, 19(7), 431-446. doi: 10.1089/10445490050085924
Lin, R. J., Chang, B. L., Yu, H. P., Liao, C. L., & Lin,
Y. L. (2006). Blocking of interferon-induced Jak-Stat signaling by Japanese
encephalitis virus NS5 through a protein tyrosine phosphatase-mediated
mechanism. Journal of virology, 80(12), 5908-5918. doi: 10.1128/JVI.02714-05
Liu, W. J., Wang, X. J., Mokhonov, V. V., Shi, P. Y.,
Randall, R., & Khromykh, A. A. (2005). Inhibition of interferon signaling
by the New York 99 strain and Kunjin subtype of West Nile virus involves
blockage of STAT1 and STAT2 activation by nonstructural proteins. Journal
of virology, 79(3), 1934-1942. doi: 10.1128/JVI.79.3.1934-1942.2005
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of
relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT
method. methods, 25(4), 402-408. doi: 10.1006/meth.2001.1262
McAllister, P. E., & Bebak, J. (1997). Infectious
pancreatic necrosis virus in the environment: relationship to effluent from
aquaculture facilities. Journal of Fish Diseases, 20(3),
201-207. doi:
10.1046/j.1365-2761.1997.00297.x
Ortega, C., Rodríguez, S., Ana, I., Romero, A.,
Monrás, M., & Enríquez, R. (2011). Evaluation of the level of Mx3 protein synthesis induced by
infectious pancreatic necrosis virus (IPNV) strains of different
infectivity. Veterinary immunology and immunopathology, 141(3-4),
190-200. doi: 10.1016/j.vetimm.2011.02.022
Ortega, S., Rodríguez, S., Espinoza, J. C., Kuznar, J.,
Romero, A., & Enríquez, R. (2014). Relationship between apoptosis and the
BH2 domain sequence of the VP5 peptide of infectious pancreatic necrosis
virus. Revista MVZ Córdoba, 19(1), 3990-4002. doi: https://doi.org/10.21897/rmvz.119
Platanias, L. C. (2005). Mechanisms of type-I-and
type-II-interferon-mediated signalling. Nature Reviews Immunology, 5(5),
375. doi: 10.1038/nri1604
Randall, R. E., & Goodbourn, S. (2008). Interferons and
viruses: an interplay between induction, signalling, antiviral responses and
virus countermeasures. Journal of General Virology, 89(1),
1-47. doi: 10.1099/vir.0.83391-0
Reed, L. J., & Muench, H. (1938). A simple method of
estimating fifty per cent endpoints. American journal of epidemiology, 27(3),
493-497. doi: https://doi.org/10.1093/oxfordjournals.aje.a118408
Robertsen, B. (2006). The interferon system of teleost
fish. Fish & shellfish immunology, 20(2), 172-191. doi: 10.1016/j.fsi.2005.01.010
Robertsen, B. (2008). Expression of interferon and
interferon-induced genes in salmonids in response to virus infection,
interferon-inducing compounds and vaccination. Fish & shellfish
immunology, 25(4), 351-357. doi: 10.1016/j.fsi.2008.02.004
Robertsen, B., Bergan, V., Røkenes, T., Larsen, R., &
Albuquerque, A. (2003). Atlantic salmon interferon genes: cloning, sequence
analysis, expression, and biological activity. Journal of Interferon
& Cytokine Research, 23(10), 601-612. doi: 10.1089/107999003322485107
Saint-Jean, S. R., & Pérez-Prieto, S. I. (2007). Effects
of salmonid fish viruses on Mx gene expression and resistance to single or dual
viral infections. Fish & shellfish immunology, 23(2),
390-400. doi: 10.1016/j.fsi.2006.11.012
Sano, M., Okamoto, N., Fukuda, H.,
Saneyoshi, M., & Sano, T. (1992). Virulence of infectious pancreatic necrosis virus is
associated with the larger RNA segment (RNA segment A). Journal of fish
Diseases, 15(4), 283-293. doi: 10.1111/j.1365-2761.1992.tb00666.x
Santi, N., Song, H., Vakharia, V. N., & Evensen, Ø.
(2005). Infectious pancreatic necrosis virus VP5 is dispensable for virulence
and persistence. Journal of virology, 79(14),
9206-9216. doi: 10.1128/JVI.79.14.9206-9216.2005
Santi, N., Vakharia, V. N., & Evensen, Ø. (2004).
Identification of putative motifs involved in the virulence of infectious
pancreatic necrosis virus. Virology, 322(1), 31-40. doi: 10.1016/j.virol.2003.12.016
Sen, G. C. (2001). Viruses and interferons. Annual
Reviews in Microbiology, 55(1), 255-281. doi:
https://doi.org/10.1146/annurev.micro.55.1.255
Shi, J., Zhang, Y. B., Liu, T. K., Sun, F., & Gui, J. F.
(2012). Subcellular localization and functional characterization of a fish IRF9
from crucian carp Carassius auratus. Fish
& shellfish immunology, 33(2), 258-266. doi: 10.1016/j.fsi.2012.05.014
Silvennoinen, O., Ihle, J. N., Schlessinger, J., & Levy,
D. E. (1993). Interferon-induced nuclear signalling by Jak protein tyrosine
kinases. Nature, 366(6455), 583. doi: 10.1038/366583a0
Sironi, J. J., & Ouchi, T. (2004). STAT1-induced
apoptosis is mediated by caspases 2, 3, and 7. Journal of Biological
Chemistry, 279(6), 4066-4074. doi: 10.1074/jbc.M307774200
Skjesol, A., Aamo, T., Hegseth, M. N., Robertsen, B., &
Jørgensen, J. B. (2009). The interplay between infectious pancreatic necrosis
virus (IPNV) and the IFN system: IFN signaling is inhibited by IPNV
infection. Virus research, 143(1), 53-60. doi:
10.1016/j.virusres.2009.03.004
Skjesol, A., Hansen, T., Shi, C. Y., Thim, H. L., & Jørgensen,
J. B. (2010). Structural and functional studies of STAT1 from Atlantic salmon (Salmo salar). BMC immunology, 11(1),
17. doi: 10.1186/1471-2172-11-17
Skjesol, A., Skjæveland, I., Elnæs, M., Timmerhaus, G.,
Fredriksen, B. N., Jørgensen, S. M., ... & Jørgensen, J. B. (2011). IPNV
with high and low virulence: host immune responses and viral mutations during
infection. Virology journal, 8(1), 396. doi: 10.1186/1743-422X-8-396
Smail, D. A., Bain, N., Bruno, D. W., King, J. A., Thompson,
F., Pendrey, D. J., ... & Cunningham, C. O. (2006). Infectious pancreatic
necrosis virus in Atlantic salmon, Salmo
salar L., post‐smolts in the Shetland Isles, Scotland: virus
identification, histopathology, immunohistochemistry and genetic comparison
with Scottish mainland isolates. Journal of Fish Diseases, 29(1),
31-41. doi: 10.1111/j.1365-2761.2005.00678.x
Song, H., Santi, N., Evensen, Ø., & Vakharia, V. N.
(2005). Molecular determinants of infectious pancreatic necrosis virus
virulence and cell culture adaptation. Journal of virology, 79(16),
10289-10299. doi: 10.1128/JVI.79.16.10289-10299.2005
Stark, G. R. (1998). I, Kerr M, Williams BR, Silverman RH,
Schreiber RD. How cells respond to interferons. Annual Review of
Biochemistry, 67, 227-264.
doi: 10.1146/annurev.biochem.67.1.227
Stein, C., Caccamo, M., Laird, G., & Leptin, M. (2007).
Conservation and divergence of gene families encoding components of innate
immune response systems in zebrafish. Genome biology, 8(11),
R251. doi: 10.1186/gb-2007-8-11-r251
Sun, B., Robertsen, B., Wang, Z., & Liu, B. (2009).
Identification of an Atlantic salmon IFN multigene cluster encoding three IFN
subtypes with very different expression properties. Developmental &
Comparative Immunology, 33(4), 547-558. doi: 10.1016/j.dci.2008.10.001
Thomas, M., Finnegan, C. E., Rogers, K. M. A., Purcell, J.
W., Trimble, A., Johnston, P. G., & Boland, M. P. (2004). STAT1: a
modulator of chemotherapy-induced apoptosis. Cancer research, 64(22),
8357-8364. doi: 10.1158/0008-5472.CAN-04-1864
Townsend, P. A., Scarabelli, T. M., Davidson, S. M., Knight,
R. A., Latchman, D. S., & Stephanou, A. (2004). STAT-1 interacts with p53
to enhance DNA damage-induced apoptosis. Journal of Biological
Chemistry, 279(7), 5811-5820. doi: 10.1074/jbc.M302637200
Verrier, E. R., Langevin, C., Benmansour, A., & Boudinot,
P. (2011). Early antiviral response and virus-induced genes in fish. Developmental
& Comparative Immunology, 35(12), 1204-1214. doi: 10.1016/j.dci.2011.03.012
Williams, K., Blake, S., Sweeney, A., Singer, J. T., &
Nicholson, B. L. (1999). Multiplex reverse transcriptase PCR assay for
simultaneous detection of three fish viruses. Journal of Clinical
Microbiology, 37(12), 4139-4141.
Wolf, K. (1988). Infectious pancreatic necrosis. Fish
viruses and fish diseases.
Zhou, Z., Hamming, O. J., Ank, N., Paludan, S. R., Nielsen,
A. L., & Hartmann, R. (2007). Type III interferon (IFN) induces a type I
IFN-like response in a restricted subset of cells through signaling pathways
involving both the Jak-STAT pathway and the mitogen-activated protein
kinases. Journal of virology, 81(14), 7749-7758. doi: 10.1128/JVI.02438-06