
Chemical profile of essential oils of the Costa Rican native tree Myrcianthes storkii (Myrtaceae)
Carlos
Chaverri1,2![]() & José F. Cicció1,2
& José F. Cicció1,2![]()
1. Universidad de Costa Rica, Escuela de Química, 11501-2060 San José, Costa Rica; cachaverri@gmail.com, jfciccio@gmail.com
2. Universidad de Costa Rica, Centro de Investigaciones en Productos Naturales (CIPRONA), 11501-2060 San José, Costa Rica.
Received 10-VII-2023 ● Corrected 08-IX-2023 ● Accepted 11-IX-2023
https://doi.org/10.22458/urj.v16i1.4863
|
ABSTRACT. Introduction: The genus Myrcianthes ranges from southern
Florida to Chile, including the Caribbean, and the species Myrcianthes
storkii is a shrub or tree found in Costa Rica and western Panama, in wet
to very rainy, cloud, and oak forests (altitude 1300-3150m). Objective:
To identify the chemical composition of essential oils from leaves, floral
buds, and twigs of M. storkii of Costa Rica. Methods: We
obtained the essential oils through hydrodistillation in a Clevenger-type
apparatus. The chemical composition of the oils was done by GC/FID and GC/MS,
using the retention indices on DB-5 and Carbowax types of capillary columns
in addition to mass spectra. Results: The oils consisted mainly of
terpenoids (55,45-87,75%). A total of 281 compounds accounted for
91,27-74,56% of the total amount of oils. The major constituents from the
leaf oil were myrcene (17,44%), cis-calamenene (12,60%), α-pinene
(5,48%), (E)-caryophyllene (5,16%), limonene (3,91%), p-cymene
(3,71%), 1,8-cineole (2,80%), and α-humulene (2,80%). The floral bud
essential oil consisted mainly of α-pinene (15,23%), cis-calamenene
(12,70%), myrcene (8,59%), 1,8-cineole (4,26%), germacrene B (3,65%), α-humulene
(3,55%), and (E)-caryophyllene oxide (2,93%). The major components of
twig oil were cis-calamenene (11,31%), palmitic acid (7,99%), (E)-caryophyllene
(4,68%),
Keywords: Myrcianthes storkii, essential oils, cis-calamenene, myrcene, α-pinene, 1,8-cineole.
|
RESUMEN. “Perfil químico de los aceites esenciales del árbol nativo costarricense Myrcianthes storkii (Myrtaceae)”. Introducción: El género Myrcianthes se extiende desde el sur de Florida hasta Chile, incluyendo el Caribe, y la especie Myrcianthes storkii es un arbusto o árbol que se encuentra en Costa Rica y el oeste de Panamá, en bosques húmedos, muy lluviosos, de neblina y robles (altitud de 1300 a 3150 m). Objetivo: Identificar la composición química de los aceites esenciales de las hojas, yemas florales y ramas de M. storkii de Costa Rica. Métodos: Obtuvimos los aceites esenciales mediante hidrodestilación en un aparato de tipo Clevenger. Determinamos la composición química de los aceites mediante cromatografía de gases con detector de ionización de llama (GC/FID) y cromatografía de gases acoplada a espectrometría de masas (GC/MS). Usamos los índices de retención en columnas capilares de tipos DB-5 y Carbowax, además de espectros de masas. Resultados: Los aceites están compuestos principalmente por terpenoides (55,45-87,75%). Identificamos 281 compuestos, que representaron el 91,27-74,56% de la cantidad total de los aceites. Los principales constituyentes del aceite de hoja fueron mirceno (17,44%), cis-calameneno (12,60%), α-pineno (5,48%), (E)-cariofileno (5,16%), limoneno (3,91%), p-cimeno (3,71%), 1,8-cineol (2,80%) y α-humuleno (2,80%). El aceite esencial de las yemas florales consistió principalmente en α-pineno (15,23%), cis-calameneno (12,70%), mirceno (8,59%), 1,8-cineol (4,26%), germacreno B (3,65%), α-humuleno (3,55%) y óxido de (E)-cariofileno (2,93%). Los componentes principales del aceite de las ramas fueron cis-calameneno (11,31%), ácido palmítico (7,99%), (E)-cariofileno (4,68%), δ-cadineno (3,28%), cubenol (3,24%) y óxido de (Z)-cariofileno (2,94%). Conclusión: La presencia de una cantidad significativa de mirceno y cis-calameneno parece ser característica de esta especie.
Palabras clave: Myrcianthes storkii, aceites esenciales, cis-calameneno, mirceno, α-pineno, 1,8-cineol.
|
Myrcianthes O. Berg is a genus composed of 39 recognized species ranging from southern Florida and Mexico to Bolivia and northern Argentina, Uruguay and north-central Chile and the Caribbean (McVaugh, 1963; Tucker et al., 1992; Tucker et al., 2002, World Flora Online [WFO], 2023). Myrcianthes storkii (Standl.) McVaugh [Synonyms: Eugenia rigidissima Cufod.; E. storkii Standl.; Myrcianthes rigidissima (Cufod.) W.D. Stevens] is a native shrub or tree of about 4 to 30m tall, with a distributional range from Costa Rica and western Panama. In Costa Rica, it is distributed in wet to very rainy, cloud, and oak forests, from 1300 to 3150m of elevation and it is known vernacularly as ‘guayabillo’ (Barrie, 2007). These forests can be found on mountain slopes, varying in the intensity of rainfall. The leaves are elliptic or obovate to broadly elliptic or broadly obovate, coriaceous, and glabrous on both sides. When the leaves are crushed, they give off a scent with aromatic flavor. Young twigs are coarsely sericeous.
Many studies on the chemical composition of essential oils of diverse species of Myrcianthes have been reported. Some of these studies are summarized in Table 1 in Appendix. The species and the morphological part from which the studied essential oil was isolated, the location, and the major compounds that constitute the oils are indicated. In general terms, the studied oils are constituted mainly of terpenes and terpenoids.
There is no information about possible traditional uses of M. storkii.
To the best of our knowledge, no previous reports on the chemical composition of essential oils of this species have been published.
MATERIALS AND METHODS
Plant materials: We collected leaves, floral buds, and twigs of Myrcianthes storkii from a single tree in the locality of Pacayas de Alvarado, Province of Cartago, Costa Rica (09°55'03"N 83°48'29"W, at an elevation of 1 700m). A voucher specimen is Luis J. Poveda Álvarez 4915 (F).
Extraction of essential oils: We isolated the oils from fresh plant material by hydrodistillation at atmospheric pressure, for 3 h using a Clevenger-type apparatus. The distilled oils were collected and dried over anhydrous sodium sulfate, filtered, and stored between 0°C and 10°C in the dark, until further analysis. The essential oil yields (v/w) were 0,05% (leaves), 0,09% (floral buds), and (0,01% twigs).
Gas chromatographic analyses (GC-FID): We analyzed the essential oils of M. storkii by capillary gas chromatography with a flame ionization detector (GC/FID) using a Shimadzu GC-2014 gas chromatograph. Data have been collected on a poly (5% diphenyl/95% dimethylsiloxane) fused silica capillary column (30m x 0,25mm; film thickness 0,25μm), (MDN-5S, Supelco). The GC integrations were performed with LabSolutions, Shimadzu GCsolution™ Chromatography Data System software, version 2.3. Operating conditions used were carrier gas N2, flow 1,0mL/min; oven temperature program: 60 to 280°C at 3°C/min, 280°C (2 min); sample injection port temperature 250°C; detector temperature 280°C; split 1:60.
Gas chromatography-mass spectrometry (GC-MS): GC-MS analyses were performed with a Shimadzu GC-2010 Plus gas chromatograph coupled with a GCMS-QP2010 SE apparatus and with GCMSsolution™ software (version 4.20), with NIST and Wiley 139 computerized databases. The analyzes were performed with two fused-silica-capillary columns with stationary phases of different polarities: 1,4-bis(dimethylsiloxy) phenylene dimethylpolysiloxane and polyethylene glycol. The data were obtained with a non-polar SLB™-5ms (Supelco) fused silica column (30m x 0,25mm; film thickness 0,25μm). Operating conditions were: carrier gas He, flow 1,4 mL min-1 with constant pressure; oven temperature was programmed linearly from 60°C to 280°C at 3°C min-1; sample injection port temperature 250°C; interface temperature 260°C; ionization voltage: 70 eV; ionization current 60μA; scanning speed 0,30s over 35 to 400 amu range; split 1:70. Also, the data were obtained with a second polar Supelcowax™10 (Supelco) fused silica column (30m x 0,25mm; film thickness 0,2 μm). Operating conditions were carrier gas He, flow 1,4mL min-1; oven temperature program: 60–220°C at 3°C min-1; sample injection port temperature 240°C; transfer line temperature 230°C; ionization voltage: 70 eV; ionization current 60 μA; scanning speed 0,30s over acquisition mass range, 35 to 400 amu; split 1:70.
Compound identification: We identified the essential oil constituents by comparison of their linear retention indices which were calculated in relation to a homologous series of n-alkanes, on a poly (5% diphenyl/95% dimethylsiloxane) type column (van den Dool & Kratz, 1963) and on polyethylene glycol capillary column and, by comparison of their mass spectra with those published in the literature (Adams, 2007), or those of our own homemade MS library, or comparing their mass spectra with those available in the computerized databases (NIST 107 and Wiley 139) or in a web source (Wallace, 2021). To obtain the retention indices for each peak, 0,1 μL of an n-alkane mixture (Sigma, C8–C32 standard mixture) was co-injected under the same experimental conditions reported above. Integration of the total chromatogram (GC/FID), expressed as area percent, without correction factors, has been used to obtain quantitative compositional data.
RESULTS
The essential oils from different parts of Myrcianthes storkii presented a complex mixture of compounds. The constituents identified, their experimental retention indices on two columns of diverse polarity, their relative percentage concentrations, and the methods used for their identification are presented in Table 2 in Appendix. The constituents are listed in order of elution on a non-polar poly-(5% phenyl 95% dimethylsiloxane) type column and for comparison purposes, previously published values of the retention indices are included (Adams, 2007; Wallace, 2021).
Myrcianthes storkii gave essential oils that were predominantly terpenoid in nature. The leaf and floral bud oils were dominated by monoterpenoids (42,66% and 38,63%, respectively) and sesquiterpenoids (44,67% and 44,69%, respectively), whereas twig oil was dominated by sesquiterpenoids (46,45%) and aliphatic compounds (18,77%). From the hydrodistilled oils, a total of 281 compounds were identified using GC/FID and GC/MS, accounting for 91,27% (leaves), 86,65% (floral buds), and 74,56% (twigs) of the total composition of the essential oils.
The leaf essential oil consisted largely of monoterpene hydrocarbons (36,98%) and sesquiterpene hydrocarbons (34,06%) with minor amounts of oxygenated derivatives. The main constituents were myrcene (17,44%), cis-calamenene (12,60%), α-pinene (5,48%), (E)-caryophyllene (5,16%), limonene (3,91%), p-cymene (3,71%), 1,8-cineole (2,80%), α-humulene (2,80%), cubenol (2,45%), α-copaene (2,22%), α-cubebene (2,10%), linalool (2,05%), (E)-caryophyllene oxide (2,04%), and β-phellandrene (2,00%). [See the total ion chromatogram (TIC) in Fig 1]. The chemical structures of some of these compounds are shown in Fig. 3.
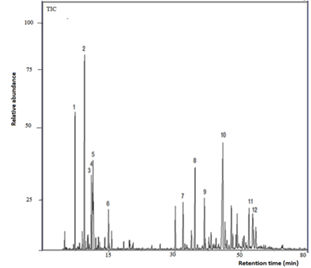
Fig. 1. GC-MS chromatogram (TIC) of Myrcianthes storkii leaf essential oil [1. α-pinene; 2. myrcene; 3. p-cymene; 4. limonene; 5. 1,8-cineole; 6. linalool; 7. α-copaene; 8. (E)-caryophyllene; 9. α-humulene; 10. cis-calamenene; 11. 1-epi-cubenol; 12. cubenol].
The composition of the floral bud essential oil also was dominated by sesquiterpene hydrocarbons (32,36%), and monoterpene hydrocarbons (30,75%) with α-pinene (15,23%), cis-calamenene (12,70%), myrcene (8,59%), 1,8-cineole (4,26%), germacrene B (3,65%), α-humulene (3,55%), (E)-caryophyllene oxide (2,93%), α-copaene (2,24%), hinesol (2,16%), and α-cubebene (2,14%) as main constituents.
The twig
essential oil was constituted mainly of sesquiterpenoids (46,45%) and aliphatic
compounds (18,77%), with a minor quantity of monoterpenoids (6,84%). The major
compounds found were cis-calamenene (11,31%),
hexadecanoic acid (7,99%), (E)-caryophyllene (4,68%), ![]() -cadinene
(3,28%), cubenol (3,24%), (Z)-caryophyllene oxide (2,94%), 1-epi-cubenol
(2,45%), α-humulene (2,38%), and α-copaene (2,19%). The aliphatic mixture of
compounds was constituted of acids (palmitic acid as the main compound),
aldehydes, alcohols, esters, and hydrocarbons. [See the total ion chromatogram
(TIC) in Fig 2].
-cadinene
(3,28%), cubenol (3,24%), (Z)-caryophyllene oxide (2,94%), 1-epi-cubenol
(2,45%), α-humulene (2,38%), and α-copaene (2,19%). The aliphatic mixture of
compounds was constituted of acids (palmitic acid as the main compound),
aldehydes, alcohols, esters, and hydrocarbons. [See the total ion chromatogram
(TIC) in Fig 2].
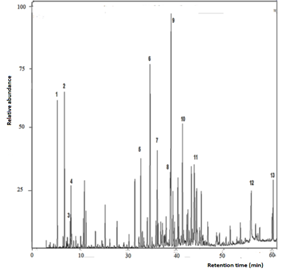
Fig. 2. GC-MS chromatogram (TIC) of Myrcianthes storkii twig essential oil [1. α-pinene; 2. myrcene; 3. p-cymene; 4. limonene; 5. α-copaene; 6. (E)-caryophyllene; 7. α-humulene; 8. δ-cadinene; 9. cis-calamenene; 10. (E)-caryophyllene oxide; 11. cubenol; 12. palmitic acid; 13. (E)-phytol].

Fig. 3. Structures of some constituents of the essential oils of Myrcianthes storkii from Costa Rica.
DISCUSSION
Analyzing the data in Table 1, the chemical composition of essential oils obtained from leaves of Myrcianthes is very varied. However, there seem to be some common, widespread compounds, such as the monoterpenes α-pinene, β-pinene, p-cymene, and limonene; the sesquiterpenes (E)-caryophyllene and α-humulene, and the terpenoids 1,8-cineole, linalool, terpinen-4-ol, α-terpineol, and (E)-caryophyllene oxide. Some of them are ubiquitous natural products that display ecological roles such as assisting in pollinator attraction, deterrent action against certain herbivores, and antimicrobial or allelopathic activities (Anaya et al., 2001; Gershenzon & Dudareva, 2007; Yazaki et al., 2017; Boncan et al., 2020; Escobar-Bravo et al., 2023).
Observing the data provided in Table 1, differences are found in the composition of the essential oils of samples of the same species that grow in different places. The oils of M. fragrans from Jamaica (Tucker et al., 1992) and Cuba (Pino et al., 2000) were rich in limonene, α-pinene, α-terpineol, and 1,8-cineole, whereas the essential oil from Venezuela (Mora et al., 2009) was rich in myrcene, β-caryophyllene, and other sesquiterpenoids. The oil from Ecuador (Armijos et al., 2018) differed from all the other samples and species in that it contained large amounts of geranial and neral. The two Costa Rican samples of this species gave oils with the unique fact of presenting as main compounds the benzenoid 1,3,5-trimethoxybenzene and (E)-methyl isoeugenol (Cole et al., 2008) and the phenylpropanoid ester, methyl (E)-cinnamate (Chaverri & Cicció, 2017).
The essential oil of M. storkii leaves is mainly constituted of terpenoids (87,75%) and small amounts of aliphatic compounds (2,55%) and benzenoids (0,91%). This oil is characterized by the dominant compounds myrcene (17,44%) and cis-calamenene (12,60%). In the studies conducted to date, only the oils of M. rhopaloides and M. leucoxyla from Colombia (Silva et al., 2016; Quijano-Célis et al., 2016), and M. fragrans from Venezuela (Mora et al., 2009) presented myrcene in significant quantities (17,7%, 17,4% and 8,9% respectively). Myrcene possesses sedative and anxiolytic properties (Rao et al., 1990), anti-inflammatory (Rufino et al., 2015), as well as antioxidant and cytoprotective properties (Xanthis et al., 2021); it also has anti-aging properties (Surendran et al., 2021) and anti-invasive activity on a human breast cancer epithelial cell line, MDA-MB-231 (Lee et al., 2015). This compound is a valuable renewable material for the industrially sustainable synthesis of many fine chemical products, which have high added value and are used in multiple applications (Behr & Johnen, 2009).
cis-Calamenene appears to be a distinctive compound in the essential oils of M. storkii from Costa Rica, accompanied by a large amount of myrcene. Of the studied species, only M. rhopaloides from Costa Rica (Cole et al., 2008) and M. myrsinoides from Ecuador (Montalván et al., 2018) presented significant amounts of the diastereoisomer, trans-calamenene (2,5% and 15,9% respectively). The cis-calamenene, an aromatic cadinene, is a major constituent (2,1-9,1%) of the essential oil of Cupressus bakeri Jeps. (Cupressaceae) foliage (Rafii et al., 1992; Kim et al., 1994) and is present in the commercial Baccharis dracunculifolia DC. essential oil (1,0%) (Weyerstahl et al., 1996). Also, this compound was identified in cuticular waxes of the stingless bees Nannotrigona testaceicornis and Plebeia droryana (Pianaro et al., 2009).
In summary, we have shown, for the first time, the chemical composition of Myrcianthes storkii essential oil from different morphological parts (leaves, flower buds, and twigs). The presence of a high amount of myrcene and cis-calamenene in the essential oils seems to be characteristic of this species.
ACKNOWLEDGEMENTS
The authors are grateful to Vicerrectoría de Investigación (UCR) for financial support (Project No. 809- B 9-170) and to Luis J. Poveda Álvarez (earlier in Herbarium JVR) for his help in the collection and identification of the species. The authors also thank the anonymous reviewers for their critical reading and valuable suggestions.
ETHICAL, CONFLICT OF INTEREST AND FINANCIAL STATEMENTS
The authors declare that they have fully complied with all pertinent ethical and legal requirements, both during the study and in the production of the manuscript; that there are no conflicts of interest of any kind; that all financial sources are fully and clearly stated in the Acknowledgements section; and that they fully agree with the final edited version of the article. A signed document has been filed in the journal archives.
REFERENCES
Adams, R. P. (2007). Identification of Essential Oil Components by Gas Chromatography/ Quadrupole Mass Spectrometry, (4th ed.). Allured Publishig Corporation.
Anaya, A. L., Espinosa-García, F., Cruz-Ortega, R. (Eds.). (2001). Relaciones químicas entre organismos: aspectos básicos y perspectivas de su aplicación. Plaza y Valdés México.
Apel, M. A., Sobral, M., & Henriques, A. T. (2006). Composição química do oleo volatile de Myrcianthes nativas da região sul do Brasil. Revista Brasileira de Farmacognosia, 16(3), 402-407. https://doi.org/10.1590/S0102-695X2006000300019
Araujo, L., Rondón, M., Morillo, A., Páes, E., & Rojas-Fermín, L. (2017). Antimicrobial activity of the essential oil of Myrcianthes myrcinoides (Kunth) Grifo (Myrtaceae) collected in the Venezuelan Andes. PharmacologyOnLine, 2, 200-204. https://tinyurl.com/ynnzyf83
Armijos, C., Valarezo, E., Cartuche, L., Zaragoza, T., Finzi, P. V., Mellerio, G. G., & Vidari, G. (2018). Chemical composition and antimicrobial activity of Myrcianthes fragrans essential oil, a natural aromatizer of the traditional Ecuadorian beverage colada morada. Journal of Ethnopharmacology, 225, 319-326. https://doi.org/10.1016/j.jep.2018.07.018
Barrie, F. R. (2007). Myrtaceae. In B. E. Hammel, M. H. Grayum, C. Herrera, & N. Zamora (Eds.). Manual de Plantas de Costa Rica. Vol. 6. (pp. 728-784). Missouri Botanical Garden Press.
Behr, A., & Johnen, L. (2009). Myrcene as a natural base chemical in sustainable chemistry: a critical review. ChemSusChem: Chemistry-Sustainability-Energy-Materials, 2(12), 1072-1095.
Boncan, D. A. T., Tsang, S. S. K., Li, C., Lee, I. H. T., Lam, H.-M., Chan, T.-F., & Hui, J. H. L. (2020). Terpenes and terpenoids in plants: Interactions with environment and insects. International Journal of Molecular Sciences, 21(19), 7382. https://doi.org/10.3390/ijms21197382
Carmen, P., De La Torre, C., & Retamar, J. A. (1972). Essential oil of Myrcianthes callicoma. Essenze e Derivati Agrumari, 42, 429-432.
Chaverri, C., & Cicció, J. F. (2017). Essential oil from leaves of Myrcianthes fragrans (Myrtaceae) from Costa Rica. A new chemotype? Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas, 16(4), 385-397. https://www.redalyc.org/pdf/856/85651256004.pdf
Cole, R. A., Haber, W. A., Lawton, R. O., & Setzer, W. N. (2008). Leaf essential oil composition of three species of Myrcianthes from Monteverde, Costa Rica. Chemistry and Biodiversity, 5(7), 1327-1334. https://doi.org/10.1002/cbdv.200890120
Collin, G., Garneau, F.-X., Gagnon, H., Pichette, A., & Lavoie, S. (2010). Analysis of cymenes in essential oils: the case of Lepechinia meyeni (Walp.) Epling. Journal of Essential Oil Research, 22(4), 310-313. https://doi.org/10.1080/10412905.2010.9700333
de Jesús, R. A., de Oliveira, H. L. M., Bortolucci, W. de C., Campo, C. F. de A. A., Faria, M. G. I., Goncalves, J. E., Colauto, N. B., Gazim, Z. C. & Linde, G. A. (2021). Antioxidant and antibacterial activity of Myrcianthes pungens leaf essential oil. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas, 20(2), 147-161. https://doi.org/10.37360/blacpma.21.20.2.12
Demo, M. S., Oliva, M. M., Zunino, M. P., López, M. L., & Zygadlo, J. A. (2002). Aromatic plants from Yungas. Part IV: Composition and antimicrobial activity of Myrcianthes pseudo-mato essential oil. Pharmaceutical Biology, 40(7), 481-484. https://doi.org/10.1076/phbi.40.7.481.14689
Escobar-Bravo, R., Lin, P-A., Waterman, J. M., & Erb, M. (2023). Dynamic environmental interactions shaped by vegetative plant volatiles. Natural Product Reports, 40, 840-865. https://doi.org/10.1039/d2np00061j
Gershenzon, J., & Dudareva, N. (2007). The function of terpene natural products in the natural world. Nature Chemical Biology, 3(7), 408-414. https://doi.org/10.1038/nchembio.2007.5
Granados, C., Yáñez, X., & Acevedo, D. (2014). Evaluación de la actividad antioxidante del aceite esencial foliar de Myrcianthes leucoxyla de norte de Santander (Colombia). Información Tecnológica, 25(3), 11-16. https://doi.org/10.4067/S0718-07642014000300003
Kim, Y-K., Cool, L. G., & Zavarin, E. cis-Calamenene-related sesquiterpenoids from Cupressus bakeri foliage. Phytochemistry, 36(4), 961-965.
Lee, J.-H., Lee, K., Lee, D.H., Shin, S. Y., Yong, Y., & Lee, Y. H. (2015). Anti-invasive effect of β-myrcene, a component of the essential oil from Pinus koraiensis cones, in metastatic MDA-MB-231 human breast cancer cells. Journal of the Korean Society for Applied Biological Chemistry, 58(4), 563-569. https://doi.org/10.1007/s13765-015-0081-3
Lopez, J. B., Jean, F. I., Gagnon, H., Collin, G., Garneau, F.X., & Pichette, A. (2005). Essential Oils from Bolivia. VII. Myrtaceae: Myrcianthes osteomeloides (Rusby) McVaugh and Myrcianthes pseudomato (Legrand) McVaugh. Journal of Essential Oil Research, 17(1), 64-65. https://doi.org/10.1080/10412905.2005.9698832
Lorenzo, D., Dellacassa, E., Bonaccorsi, I., & Mondello, L. (2001). Uruguayan essential oils. Composition of leaf oil of Myrcianthes cisplatensis (Camb.) Berg. ("Guayabo colorado") (Myrtaceae). Flavour and Fragrance Journal, 16(2), 97-99. https://doi.org/10.1002/1099-1026(200103/04)16:2<97::AID-FFJ952>3.0.CO;2-C
Malagón, O., Vila, R., Iglesias, J., Zaragoza, T., & Cañigueral, S. (2003). Composition of the essential oils of four medicinal plants from Ecuador. Flavour and Fragrance Journal, 18(6), 527-531. https://doi.org/10.1002/ffj.1262
Marin, R., Apel, M. A., Limberger, R. P., Raseira, M. C. B., Pereira, J. F. M., Zuanazzi, J. A. S., & Henriques, A. T. (2008). Volatile components and antioxidant activity from some myrtaceous fruits cultivated in Southern Brazil. Latin American Journal of Pharmacy, 27(2), 172-177. https://tinyurl.com/yo24maef
McVaugh, R. (1963). Tropical American Myrtaceae, II. Notes on generic concepts and descritions of previously unrecognized species. Fieldiana Botany, 29(8), 395-532. http://tinyurl.com/yp7k7hkh
Montalván, M., Peñafiel, M. A., Ramírez, J., Cumbicus, N., Bec, N., Larroque, C., Bicchi, C., & Gilardoni, G. (2019). Chemical composition, enantiomeric distribution, and sensory evaluation of the essential oils distilled from the Ecuadorian species Myrcianthes myrcinoides (Kunth) Grifo and Myrcia mollis (Kunth) DC. (Myrtaceae). Plants, 8, 511. https://doi.org/10.3390/plants8110511
Mora, V. F. D., Rojas, L. B., Usubillaga, A., Carmona, J., & Silva, B. (2009). Composición química del aceite esencial de Myrcianthes fragrans (Sw.) McVaugh de los Andes venezolanos. Revista de la Facultad de Farmacia, 51(1), 20-23.
Pianaro, A., Menezes, C., Kerr, W. E., Singer, R. B., Patricio, E. F., Marsaioli, A. J. (2009). Stingless bees: Chemical differences and potential functions in Nannotrigona testaceicornis and Plebeia droryana males and workers. Journal of Chemical Ecology, 35, 1117-1128. https://doi.org/10.1007/s10886-009-9679-4
Pino, J. A., Rosado, A., Bello, A., Urquiola, A., & García, G. (2000). Essential oil of Myrcianthes fragrans (Sw.) McVaugh from Cuba. Journal of Essential Oil Research, 12(2), 225-226. https://doi.org/10.1080/10412905.2000.9699503
Pombo, L. M., Borrego, P., Matulevich, J. Teheran, A. A., & Barajas, L. (2016). Composition and antimicrobial activity of the essential oils of three plant species from the Sabana of Bogotá (Colombia): Myrcianthes leucoxyla, Vallea stipularis and Phyllanthus salviifolius. Natural Products Communications, 11(12), 1913-1918. https://doi.org/10.1177/1934578X1601101234
Quijano-Célis, C., Pino, J. A., Echeverri, D., & Morales, G. (2016). Essential oil of Myrcianthes leucoxyla (Ortega) McVaugh leaves from Colombia. Journal of Essential Oil Bearing Plants, 19(6), 1510-1515. https://doi.org/10.1080/0972060X.2016.1224687
Rafii, Z., Cool, L. G., & Zavarin, E. (1992). Variability of foliar mono- and sesquiterpenoids of Cupressus bakeri. Biochemical Systematics and Ecology, 20(2), 123-131.
Rao, V. S., Menezes, A. M., & Viana, G. S. (1990). Effect of myrcene on nociception in mice. Journal of Pharmacy and Pharmacology, 42(12), 877-878. https://doi.org/10.1111/j.2042-7158.1990.tb07046.x
Romanenko, E. P. & Tkachev, A. V. (2006). Identification by GC-MS of cymene isomers and 3,7,7-trimethylcyclohepta-1,3,5-triene in essential oils. Chemistry of Natural Compounds, 42(6), 699-701. https://doi.org/10.1007/s10600-006-0256-6
Romero, D., Cartuche, L., Valarezo, E., Cumbicus, N., & Morocho, V. (2023). Chemical profiling, anticholinesterase, antioxidant, and antibacterial potential of the essential oil from Myrcianthes discolor (Kunth) McVaugh, an aromatic tree from Southern Ecuador. Antibiotics, 12(4), 677. https://doi.org/10.3390/antibiotics12040677
Rufino, A. T., Ribeiro, M., Sousa, C., Judas, F., Salgueiro, L., Cavaleiro, C., & Mendes, A. F. (2015). Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. European Journal of Pharmacology, 750, 141-150. https://doi.org/f64wrr
Setzer, W. N., Setzer, M. C., Moriarity, D. M., Bates, R. B., & Haber, W. A. (1999). Biological activity of the essential oil of Myrcianthes sp. nov. “Black Fruit” from Monteverde, Costa Rica. Planta Medica, 65(5), 468-469. https://doi.org/10.1055/s-2006-960816
Silva, D. A., Matulevich, J. A., & Devia, B. O. (2016). Composición química del aceite esencial de hojas de Myrcianthes rhopaloides (Kunt) McVaugh (Myrtaceae). Revista Facultad de Ciencias Básicas, 12(1), 84-91. https://doi.org/10.18359/rfcb.1857
Surendran, S., Qassadi, F., Surendran, G., Lilley, D., & Heinrich, M. (2021). Myrcene - What are the potential health benefits of this flavouring and aroma agent? Frontiers in Nutrition, 8, 699666. https://doi.org/10.3389/fnut.2021.699666
Taher, H. A., Santi de Bongioanni, M. N., & Talenti, E. C. J. (1983). Study of the essential oil of Myrcianthes cisplatensis (Cambassedes) Berg. Essenze e Derivati Agrumari , 53, 13-25.
Toloza, A. C., Zygadlo, J., Cueto, G. M., Biurrun, F., Zerba, E., & Picollo. M. I. (2006). Fumigant and repellent properties of essential oils and component compounds against permethrin-resistant Pediculus humanus capitis (Anoplura: Pediculidae) from Argentina. Journal of Medical Entomology, 43(5), 889-895. https://doi.org/10.1093/jmedent/43.5.889
Tucker, A. O., Maciarello, M. J., & Landrum, L. R. (1992). Volatile leaf oils of Caribbean Myrtaceae. VI. Myrcianthes fragrans (Sw.) McVaugh of Jamaica. Journal of Essential Oil Research, 4(3), 313-314. https://doi.org/10.1080/10412905.1992.9698071
Tucker, A. O., Maciarello, M. J., & Landrum, L. R. (2002). Volatile leaf oil of Myrcianthes coquimbensis (Barnéoud) Landrum et Grifo (Myrtaceae) of Chile. Journal of Essential Oil Research, 14(1), 40-41. https://doi.org/10.1080/10412905.2002.9699756
Ubiergo, G., Taher, H. A., & Talenti, E. C. (1986). Mono and sesquiterpenoids from the essential oil of Myrcianthes pungens. Anales de la Asociación Química Argentina, 74, 567-569.
van den Dool, H., & Kratz, P. D. (1963). A generalization of the retention index including linear temperature programmed gas-liquid partition chromatography. Journal of Chromatography A, 11, 463-471. https://doi.org/10.1016/S0021-9673(01)80947-X
Vasconcelos, T. N. C., Proença, C. E. B., Ahmad, B., Aguilar, D. S., Aguilar, R., Amorim, B. S., Campbell, K., Costa, I. R., De-Carvalho, P. S., Faria, J. E. Q., Giaretta, A., Kooij, P. W., Lima, D. F., Mazine, F. F., Peguero, B., Prenner, G., Santos, M. F., Soewarto, J., Wingler, A., & Lucas, E. J. (2017). Myrteae phylogeny, calibration, biogeography and diversification patterns: Increased understanding in the most species rich tribe of Myrtaceae. Molecular Phylogenetics and Evolution, 109, 113-137. https://doi.org/10.1016/j.ympev.2017.01.002
Wallace, W. E. (2021). Mass spectra (by NIST Mass Spec Data Center). In P.J. Linstrom & W.G. Mallard (Eds.), Nits Chemistry WebBook: NIST Standard Reference Database Number 69. National Institute of Standards and Technology. http://webbook.nist.gov
Werka, J. S., Boehme, A. K., & Setzer, W. N. (2007). Biological activities of essential oils from Monteverde, Costa Rica. Natural Product Communications, 2(12), 1215-1219. https://doi.org/10.1177/1934578X0700201204
Weyerstahl, P., Christiansen, C., & Marschall, H. (1996). Constituents of Brazilian vassoura oil. Flavour and Fragrance Journal, 11(1), 15-23. https://doi.org/10.1002/(SICI)1099-1026(199601)11:1%3C15::AID-FFJ541%3E3.0.CO;2-H
World Flora Online (WFO). (2023). Myrcianthes O. Berg. https://tinyurl.com/yogu523b
Wilson, P. G. (2011). Myrtaceae. In Kubitzki, K. (ed.). Families and genera of vascular plants. Vol. 10. Flowering plants: Eudicots-Sapindales, Cucurbitales, Myrtaceae. Springer-Verlag.
Xanthis, V., Fitsiou, E., Voulgaridou, G. P., Bogadakis, A., Chlichlia, K., Galanis, A., & Pappa, A. (2021). Antioxidant and cytoprotective potential of the essential oil Pistacia lentiscus var. chia and its major components myrcene and α-pinene. Antioxidants, 10, 127. https://doi.org/10.3390/antiox10010127
Yáñez, X., Granados, C., Durán, M. (2013). Composición química y actividad antibacteriana del aceite esencial de Myrcianthes leucoxyla de Pamplona (Colombia). @limentech Ciencia y Tecnología Alimentaria, 11(1), 79-84. https://doi.org/10.240/16927125.v1.n1.2013.497
Yazaki, K., Arimura, G-I., Ohnishi, T. (2017). ’Hidden’ terpenoids in plants: Their biosynthesis, location and ecological roles. Plant Cell Physiology, 58(10), 1615-1621. https://doi.org/10.1093/pcp/pcx123
Zygadlo, J. A., Rotman, A. D., Perez-Alonso, M. J., & Velasco-Negueruela, A. (1997). Leaf oils of two Myrcianthes species from Argentina: M. pungens (Berg.) Legrand and M. cisplatensis (Camb.) Berg. Journal of Essential Oil Research, 9(2), 237-239. https://doi.org/10.1080/10412905.1997.9699470
APPENDIX
TABLE 1
Major compounds present in some Myrcianthes spp. essential oils.
|
Species |
Country, location |
Essential oil constituents (>2,0%) |
Biological observations |
References |
|
M. callicoma McVaugh |
Argentina |
α-Pinene, limonene, and 1,8-cineole. |
|
Carmen et al., 1972 |
|
M. cisplatensis (Camb.) Berg |
Argentina |
1,8-Cineole (13,5%), and geraniol (8,4%). |
|
Taher et al., 1983 |
|
M. cisplatensis (Camb.) Berg |
Argentina, Catamarca. (Air-dried leaves) (0,15%) |
1,8-Cineole (40,7%), limonene (22,1%), α-terpineol (7,7%), linalool (4,8%), and α-pinene (4,3%). |
|
Zygadlo et al., 1997 |
|
M. cisplatensis (Camb.) Berg |
Uruguay, ‘Cerros pelados’, Canelones. (Air-dried leaves) |
1,8-Cineole (53,8%), α-pinene (16,6%), α-terpineol (4,2%), limonene (4,1%), and thujopsan-4α-ol (2,0%). |
|
Lorenzo et al., 2001 |
|
M. cisplatensis Camb.) Berg |
Brazil, Alegrete, Rio Grande do Sul. (Fresh leaves) (0,2%) |
1,8-Cineole (29,8%), limonene (10,9%), β-caryophyllene (10,8%), α-pinene (8,9%), α-terpineol (5,7%), guaiol (4,9%), globulol (4,8%), α-selinene (2,7%), aromadendrene (2,5%), and α-humulene (2,0%). |
|
Apel et al., 2006 |
|
M. cisplatensis (Camb.) Berg |
Argentina (Dried leaves)
|
1,8-Cineole (45,7%), limonene (27,1%), α-terpineol (7,7%), linalool (4,8%), α-pinene (4,3%), and δ-cadinene (2,3%). |
Fumigant and repellent properties against permethrin-resistant head lice. |
Toloza et al., 2006 |
|
M. coquimbensis (Barnèoud) L.R. Landrum & Grifo |
Chile, La Serena. (Air-dried leaves)
|
Limonene (14,5%), carvone (8,7%), α-pinene (7,2%), β-pinene (5,7%), p-cymene (5,3%), trans-carveol (4,9%), cis-pinocarveol (4,3%), linalool (4,1%), trans-linalool oxide (furanoid) (3,6%), myrtenal (3,4%), pinocarvone (3,2%), verbenone (2,9%), cis-linalool oxide (furanoid) (2,8%), and myrtenol (2,2%). |
|
Tucker et al., 2002 |
|
M. discolor (Kunth) McVaugh |
Ecuador, Loja-Chuquiribamba Road, Loja. (Fresh leaves) (0,08%) |
β-Caryophyllene
(29,40%), bicyclogermacrene (7,45%), β-elemene (6,93%), α-cubebene (6,06%), α-humulene (3,96%), |
Strong inhibitory effect against acetylcholinesterase (AChE) and moderate antiradical effect. |
Romero et al., 2023 |
|
M. fragrans (Sw.) McVaugh |
Jamaica, Douglas Castle, St. Ann. (Air-dried leaves)
|
Limonene (56,0%), α-terpineol (10,9%), 1,8-cineole (7,1%), α-pinene (6,9%), and β-pinene (2,0%). |
|
Tucker et al., 1992 |
|
M. fragrans (Sw.) McVaugh |
Cuba, Pinar del Río. (Leaves and stalks) (1,4%) |
α-Pinene (41,8%), limonene (30,0%), 1,8-cineole (6,5%), α-terpineol (5,7%), and cis-piperityl acetate (2,1%). |
|
Pino et al., 2000 |
|
M. fragrans (Sw.) McVaugh |
Costa Rica, Monteverde. (Fresh leaves) |
(See Cole et al., 2008). |
Cytotoxic to Hep G2 and SK-Mel-28 cells. |
Werka et al., 2007 |
|
M. fragrans (Sw.) McVaugh |
Costa Rica, Monteverde. (Fresh leaves) (0,03%) |
1,3,5-Trimethoxybenzene (15,7%), α-cadinol (10,4%), (Z)-hex-3-en-1-ol (10,0%), eudesma-4(15),7-dien-1β-ol (9,0%), caryophyllene oxide (7,8%), spathulenol (7,5%), muurola-4,10(14)-dien-1β-ol (4,7%), caryophylla-4(12),8(13)-dien-5β-ol (4,2%), humulene epoxide II (3,9%), τ-muurolol (3,5%), α-muurolol (3,2%), and (E)-methylisoeugenol (2,5%). |
|
Cole et al., 2008 |
|
M. fragrans (Sw.) McVaugh |
Venezuela, Aldea Llanetes, Táchira. (Fresh leaves) (0,08%) |
β-Caryophyllene (11,5%), myrcene (8,9%), phellandrene/ limonene (8,7%), α-humulene (6,7%), α-copaen-8-ol (6,7%), globulol (4,9%), viridiflorol (4,7%), bicyclogermacrene (4,4%), α-copaene (3,5%), δ-cadinol (2,8%), δ-cadinene (2,6%), linalool (2,3%), and τ-cadinol (2,1%). |
|
Mora et al., 2009 |
|
M. fragrans (Sw.) McVaugh |
Costa Rica, Santo Domingo, Heredia (Fresh leaves) (0,5%) |
Methyl (E)-cinnamate (39,6%), limonene (34,6%), α-pinene (6,8%), linalool (6,8%), and heptan-2-ol (2,0%). |
|
Chaverri & Cicció, 2017 |
|
M. fragrans (Sw.) McVaugh |
Ecuador, Cerro Villonaco, Loja. (Aerial parts) (0,28-0,38%) |
Geranial (23,6-31,1%), neral (17,8-24,3%), β-pinene (3,9-7,5%), α-pinene (2,8-5,9%), (2E,6E)-farnesal (3,2-8,0%), (2Z,6E)-farnesal (3,0-6,7%), and geraniol (2,5-3,1%). |
Antimicrobial activity against Klebsiella pneumoniae, Candida albicans, and Saccharomyces cerevisiae |
Armijos et al., 2018 |
|
M. gigantea (D. Legrand) D. Legrand |
Brazil, Espumoso, Rio Grande do Sul. (Fresh leaves) (0,1%) |
Spathulenol (28,9%), iso-spathulenol (9,5%, α-cadinol (7,0%), caryophyllene oxide (6,7%), limonene (4,5%), α-pinene (3,5%), β-pinene (2.8%), globulol (2,8%), α-copaene (2,6%), β-selinene (2,5%), and (Z)-hex-3-en-1-ol (2,4%).
|
|
Apel et al., 2006 |
|
M. leucoxyla (Ortega) McVaugh |
Colombia, Pamplona, Santander. (Dried leaves) (0,3%) |
α-Pinene (28,4%), 1,8-cineole (15,7%), β-caryophyllene (8,8%), spathulenol (3,3%), guaiol (3,1%), β-humulene (3,0%), and caryophyllene oxide (3,0%). |
Antimicrobial activity against Staphylococcus aureus. Antioxidant activity. |
Yáñez et al., 2013. Granados et al., 2014 |
|
M. leucoxyla (Ortega) McVaugh |
Colombia, Andean Plateau, Sabana de Bogotá (Fresh leaves) (0,1%) |
Caryophyllene oxide (21,7%), α-terpineol (8.0%), linalool (7,8%), 1,8-cineole (6,3%), geraniol (5,1%), epi-globulol (3,4%), geranyl acetate (3,2%), germacrene D (3,2%), 2-carene (2,9%), and τ-cadinol (2,7%). |
Antimicrobial activity against Pseudomonas aeruginosa and Salmonella typhimurium. |
Pombo et al., 2016 |
|
M. leucoxyla (Ortega) McVaugh |
Colombia, Bogotá (Young fresh leaves) (0,1%) |
Limonene (21,2%), myrcene (17,4%), spathulenol (7,1%), β-pinene (8,4%), α-pinene (5,4%), caryophyllene oxide (2,7%), linalool (2,4%), α-terpineol (2,3%), terpinen-4-ol (2,2%), and α-cadinol (2,2%). |
|
Quijano-Célis et al., 2016 |
|
M. myrsinoides (Kunth) Grifo |
Venezuela, Mérida. (Leaves) (0,5%) |
Terpinen-4-ol (32,2%), o-cymene (8,2%), spathulenol (7,6%), caryophyllene oxide (7,1%), α-terpineol (4,1%), β-oplopenone (3,9%), limonene (3,8%), isoaromadendrene epoxide (3,8%), humulene epoxide II (3,0%), and τ-muurolol (2,5%). |
Antimicrobial activity against Bacillus cereus, B. subtilis, and Staphylococcus epidermidis. |
Araujo et al., 2017
|
|
M. myrsinoides (Kunth) Grifo |
Ecuador, Gonzanamá, Loja. (Fresh leaves) (0,3%)
|
Caryophyllene (16,6%), trans-calamenene (15,9%), 1,8-cineole (10,4%), spathulenol (6,2%), limonene (5,3%), trans-cadina-1,4-diene (3,5%), cis-muurola-4(14),5-diene (2,6%), α-pinene (2,5%), α-copaene (2,1%), germacrene D (2,1%), and α-terpineol (2,0%). |
|
Montalván et al., 2018 |
|
M. osteomeloides (Rusby) McVaugh |
Bolivia, Cochabamba. (Fresh leaves) (0,6%) |
1,8-Cineole (55,7%), α-pinene (17,9%), α-terpineol (8,5%), β-pinene (4,6%), and limonene (4,1%). |
|
López et al., 2005 |
|
M. pseudo-mato (D. Legrand) Mc.Vaugh |
Argentina, Oran, Salta. (Dried leaves) (0,3%)
|
1,8-Cineole (32,5%), β-caryophyllene (18,9%), sabinene (6,6%), α-pinene (6,5%), aromadendrene (5,4%), τ-muurolol (4,5%), (E)-nerolidol (3,5%), τ-cadinol (3,4%), spathulenol (3,3%), α-terpineol (2,7%), β-eudesmol (2,3%), and α-humulene (2,1%). |
Antimicrobial activity against Staphylococcus aureus, Bacillus cereus and Micrococcus luteus. |
Demo et al., 2002 |
|
M. pseudo-mato (D. Legrand) Mc.Vaugh |
Bolivia, Cochabamba. (Fresh leaves) (0,1%) |
1,8-Cineole (24,4%), α-pinene (17,1%), linalool (11,7%), limonene (8,5%), γ-terpinene (7,3%), p-cymene (3,9%), and α-terpineol (2,4%). |
|
López et al., 2005 |
|
M. pungens (Berg) D. Legrand |
Argentina (Leaves) |
1,8-Cineole (13,5%), pulegone (9,4%), farnesol (9,0%), nerol (5,4%), and geraniol (4,5%). |
|
Ubiergo et al., 1986 |
|
M. pungens (Berg) D. Legrand |
Argentina, Catamarca. (Air-dried leaves) (0,2%) |
1,8-Cineole (45,9%), limonene (17,3%), α-terpineol (8,1%), α-pinene (3,3%), linalool (3,0%), and globulol (2,8%). |
|
Zygadlo et al., 1997 |
|
M. pungens (O. Berg) D. Legrand |
Brazil, Viamão, Rio Grande do Sul. (Fresh leaves) (0,1%) |
β-Caryophyllene (10,1%), spathulenol (9,7%), β-elemene (9,1%), α-cadinol (8,0%), bicyclogermacrene (6,9%), globulol (6,2%), epi-globulol (4,7%), β-bisabolene (3,3%), (E)-γ-bisabolene (3,3%), β-selinene (3,1%), 1,8-cineole (2,7%), caryophyllene oxide (2,3%), α-pinene (2,1%), τ-muurolol (2,1%), α-humulene (2,0%), and δ-cadinene (2,0%). |
|
Apel et al., 2006 |
|
M. pungens (O. Berg) D. Legrand |
Brazil, Pelotas, Rio Grande do Sul. (Cultivated, fresh edible, and ripped fruits) |
β-Caryophyllene (32,7%), germacrene D (14,2%), bicyclogermacrene (11,2%), β-eudesmol (8,1%), furfural (7,7%), epi-globulol (3,9%), elemol (3,8%), α-humulene (3,3%), γ-eudesmol (2,5%), and α-eudesmol (2,5%). |
|
Marín et al., 2008 |
|
M. pungens (O. Berg) D. Legrand |
Brazil, Maringá. (Dried leaves) (0,2%)
|
β-Caryophyllene (11,7%), 1,8-cineole (10,1%), bicyclogermacrene (7,9%), 5-epi-neointermedeol (6,0%), caryophyllene oxide (5,2%), limonene (3,5%), β-selinene (3,4%), (E)-β-ocimene (3,3%), β-elemene (3,0%), δ-cadinene (3,0%), α-cubebene (2,8%), germacrene A (2,3%), and germacrene B (2,2%). |
Antimicrobial activity against Staphylococcus aureus and Bacillus cereus. |
de Jesús et al., 2021 |
|
M. rhopaloides (Kunth) McVaugh
|
Ecuador, Cerro el Villonaco, Loja. (Fresh leaves) (0,3%) |
Geranial (33,7%), neral (25,0%), β-pinene (9,0%), α-pinene (6,9%), geranyl acetate (3,0%), and geraniol (2,3%). |
|
Malagón et al., 2003 |
|
*M. rhopaloides (Kunth) McVaugh |
Costa Rica, Chomogo, Monteverde. (Fresh leaves) |
(See Cole et al., 2008). |
Cytotoxic to SK-Mel-28 cells. |
Werka et al., 2007 |
|
*M. rhopaloides (Kunth) McVaugh |
Costa Rica, Chomogo, Monteverde. (Fresh leaves) (0,02%) |
Linalool (17,7%), α-cadinol (14,4%), spathulenol (11,1%), τ-cadinol (9,6%), 1-epicubenol (6,9%), α-muurolol (5,5%), cyclocolorenone (4,9%), α-terpineol (3,5%), eudesma-4(15),7-dien-1β-ol (3,4%), caryophyllene oxide (3,3%), tetradecan-1-ol (3,3%), trans-calamenene (2,5%), and δ-cadinene (2,2%). |
|
Cole et al., 2008 |
|
*M. rhopaloides (Kunth) McVaugh |
Costa Rica, Brillante, Monteverde. (Fresh leaves) |
(E)-Hex-2-enal (46,1%), 1,8-cineole (12,5%), linalool (9,1%), α-cadinol (6,7%), α-terpineol (4,4%), τ-muurolol (2,6%), and terpinen-4-ol (2,0%). |
|
Cole et al., 2008 |
|
M. rhopaloides (Kunth) McVaugh |
Colombia, Macheta, Cundinamarca (Fresh leaves) (0,28%) |
Citronelal (27,3%), myrcene (17,7%), citronelol (15,5%), neoisopulegol (6,6%), α-pinene (4,2%), β-pinene (4,2%), β-caryophyllene (2,5%), isopulegol (2,3%), and α-farnesene (2,2%). |
|
Silva et al., 2016 |
|
M. sp. nov. ‘black fruit’ |
Costa Rica, Monteverde. (Fresh leaves) |
1,8-Cineole (52,8%), α-pinene (11,8%), α-terpineol (11,7%), heptan-2-ol (11,1%), β-pinene (8,4%), and limonene (4,3%). |
In vitro citotoxic activity against Hep-G2 and SK-Mel-28 human tumor cell lines. |
Setzer et al., 1999 |
|
M. sp. nov. ‘black fruit’ |
Costa Rica, Monteverde. (Fresh leaves) |
1,8-Cineole (38,3%), α-terpineol (21,2%), heptan-2-ol (15,5%), terpinen-4-ol (4,2%), and β-pinene (3,8%). |
|
Cole et al., 2008 |
* According to Manual de Plantas de Costa Rica, vol. 6, M. rhopaloides (Kunth) McVaugh does not inhabit Costa Rica, and this name could probably have been used instead of M. storkii (?) (Barrie, 2007, p. 770).
TABLE 2
Chemical constituents of the essential oils of Myrcianthes storkii from Costa Rica
|
aCompound |
bRILit |
cRIExp |
dSw10Exp |
Class |
(L) Leaf (%) |
(F) Floral buds (%) |
(T) Twigs (%) |
eIM |
|
3-Methylbut-2-enal |
790 |
|
1 206(F) |
A |
|
ftr |
|
2;3 |
|
Hexanal |
801 |
|
1 084(L,F) |
A |
tr |
tr |
tr |
2;3 |
|
(E)-Hex-2-enal |
846 |
841 |
1 222(L) |
A |
0,10 |
0,01 |
|
1;2;3 |
|
(Z)-Hex-3-enol |
850 |
850 |
1 384(F) |
A |
0,65 |
0,08 |
|
1;2;3 |
|
(E)-Hex-2-enol |
854 |
853 |
|
A |
|
tr |
|
1;3 |
|
Hexan-1-ol |
863 |
863 |
1 352(L,F) |
A |
0,11 |
0,02 |
0,03 |
1;2;3;4 |
|
2-Butyl furan |
885 |
882 |
|
Misc. |
|
tr |
|
1;3 |
|
Heptan-2-one |
889 |
888 |
|
A |
|
tr |
|
1;3 |
|
Nonane |
900 |
900 |
|
A |
|
|
0,02 |
1;3 |
|
Bornylene (2-Bornane) |
908 |
|
1 512(T) |
M |
|
|
tr |
2;3 |
|
Heptanal |
901 |
900 |
1 189(T) |
A |
0,04 |
tr |
0,02 |
|
|
Anisole |
913 |
914 |
|
B |
|
|
0,03 |
1;3 |
|
Tricyclene |
921 |
920 |
|
M |
0,02 |
|
|
1;3 |
|
Cumene |
924 |
924 |
1 177(L) |
B |
tr |
|
|
1;2;3 |
|
α-Thujene |
924 |
926 |
|
M |
0,15 |
0,16 |
0,02 |
1;3 |
|
3,5-Dimethylene-1,4,4-trimethylcyclopentene |
931 |
|
1 179(F) |
IT |
|
tr |
|
2;3 |
|
α-Pinene |
932 |
933 |
1 029(L,F,T) |
M |
5,48 |
15,23 |
1,57 |
1;2;3;4 |
|
α-Fenchene |
945 |
944 |
1 056(L,F) |
M |
0,04 |
tr |
|
1;2;3 |
|
Camphene |
946 |
945 |
1 068(L,F) |
M |
tr |
0,13 |
|
1;2;3 |
|
(E)-Hept-2-enal |
947 |
946 |
|
A |
0,03 |
|
tr |
1;3 |
|
Thuja-2,4(10)-diene |
953 |
953 |
1 128(L,F) |
M |
tr |
0,03 |
|
1;2;3 |
|
Isobutyl butanoate |
958 |
961 |
1 159(F) |
A |
|
tr |
|
1;2;3 |
|
Sabinene |
969 |
972 |
1 123(F) |
M |
|
0,11 |
|
1;2;3 |
|
Oct-1-en-3-one |
972 |
973 |
|
A |
0,03 |
|
tr |
1;3 |
|
β-Pinene |
974 |
977 |
1 112(L,F,T) |
M |
0,64 |
0,88 |
0,15 |
1;2;3;4 |
|
Octan-3-one |
979 |
979 |
|
A |
|
|
tr |
1;3 |
|
2-Pentylfuran |
984 |
984 |
1 232(L,T) |
Misc. |
tr |
|
tr |
1;2;3 |
|
6-Methylhept-5-en-2-one |
987 |
985 |
1 337(F) |
A |
|
tr |
|
1;2;3 |
|
Myrcene |
988 |
990 |
1 168(L,T) |
M |
17,44 |
8,59 |
1,78 |
1;2;3 |
|
Octanal |
998 |
995 |
1 292(L,T) |
A |
0,18 |
|
0,06 |
1;2;3 |
|
δ-2-Carene |
1 001 |
1 001 |
1 138(F) |
M |
|
tr |
|
1;2;3 |
|
(E)-Hex-3-enyl acetate |
1 001 |
1 000 |
|
A |
0,06 |
|
|
1;3 |
|
α-Phellandrene |
1 002 |
1 006 |
1 175(T) |
M |
0,46 |
0,63 |
0,13 |
1;2;3 |
|
p-Mentha-1(7),8-diene |
1 003 |
|
1 172(L,F) |
M |
tr |
tr |
|
2;3 |
|
δ-3-Carene |
1 008 |
1 011 |
1 149(L,F,T) |
M |
0,66 |
0,45 |
0,12 |
1;2;3 |
|
α-Terpinene |
1 014 |
1 017 |
1 182(L,T) |
M |
0,13 |
0,14 |
0,05 |
1;2;3 |
|
m-Cymene* |
1 020 |
1 021 |
1 272(L,F,T) |
M |
tr |
tr |
0,43 |
1;2;3 |
|
p-Cymene* |
1 022 |
1 024 |
|
M |
3,71 |
1,59 |
|
1;3 |
|
Limonene |
1 024 |
1 029 |
1 204(L,F,T) |
M |
3,91 |
0,10 |
0,87 |
1;2;3;4 |
|
β-Phellandrene |
1 025 |
1 030 |
1 212(L,T) |
M |
2,00 |
0,10 |
0,37 |
1;2;3 |
|
1,8-Cineole |
1 026 |
1 031 |
1 211(L) |
OM |
2,80 |
4,26 |
0,09 |
1;2;3;4 |
|
(Z)-β-Ocimene |
1 032 |
1 035 |
1 235(L,T) |
M |
0,94 |
1,50 |
0,18 |
1;2;3 |
|
Phenyl acetaldehyde |
1 041 |
1 041 |
|
B |
|
|
0,05 |
1;3 |
|
(E)-β-Ocimene |
1 044 |
1 045 |
1 252(L,T) |
M |
0,43 |
0,22 |
0,09 |
1;2;3 |
|
(E)-Oct-2-enal |
1 049 |
|
1 427(L) |
A |
tr |
|
|
2;3 |
|
Isopentyl butanoate |
1 052 |
1 056 |
1 270(L,F) |
A |
tr |
tr |
|
1;2;3 |
|
γ-Terpinene |
1 054 |
1 058 |
1 245(L,T) |
M |
0,44 |
0,32 |
0,19 |
1;2;3 |
|
(E)-Oct-2-en-1-ol |
1 060 |
1 064 |
|
A |
tr |
|
|
1;3 |
|
Octan-1-ol |
1 063 |
1 065 |
1 559(L,F) |
A |
0,02 |
0,02 |
0,05 |
1;2;3 |
|
cis-Linalool oxide (Furanoid) |
1 067 |
|
1 439(F) |
OM |
|
tr |
|
2;3 |
|
p-Cresol |
1 071 |
1 071 |
|
B |
|
0,02 |
|
1;3 |
|
4-Pentenyl butanoate |
1 076 |
1 075 |
1 341(L) |
A |
tr |
0,32 |
|
1;2;3 |
|
m-Cymenene |
1 082 |
1 083 |
1 420(L,F) |
M |
tr |
tr |
|
1;2;3 |
|
trans-Linalool oxide (Furanoid) |
1 084 |
|
1 468(F) |
OM |
|
tr |
|
2;3 |
|
p-Mentha-2,4(8)-diene |
1 085 |
1 085 |
|
M |
0,02 |
tr |
|
1;3 |
|
Terpinolene |
1 086 |
1 089 |
1 1282(T) |
M |
0,48 |
0,51 |
0,20 |
1;2;3 |
|
Methyl benzoate |
1 088 |
1 091 |
|
B |
|
tr |
|
1;3 |
|
p-Cymenene |
1 089 |
1 091 |
1 484(L,F) |
M |
0,03 |
tr |
0,05 |
1;2;3 |
|
6,7-Epoximyrcene |
1 090 |
|
1 410(L,F) |
OM |
tr |
tr |
|
2;3 |
|
Linalool |
1 095 |
1 097 |
1 552(L,F,T) |
OM |
2,05 |
2,65 |
0,37 |
1;2;3;4 |
|
Undecane |
1 100 |
|
1 100(L) |
A |
tr |
|
|
2;3;4 |
|
Nonanal |
1 100 |
1 104 |
1 394(L,T) |
A |
0,22 |
tr |
0,80 |
1;2;3 |
|
Perillene |
1 102 |
1 105 |
1 420(L,T) |
Misc. |
tr |
0,10 |
0,05 |
1;2;3 |
|
1,3,8-Menthatriene |
1 108 |
|
1 218(F) |
M |
|
tr |
|
2;3 |
|
3-Methyl-3-butenyl isovalerate |
1 112 |
1 114 |
|
A |
|
0,06 |
|
1;3 |
|
exo-Fenchol |
1 118 |
1 120 |
|
OM |
|
0,05 |
|
1;3 |
|
cis-p-Menth-2-en-1-ol |
1 118 |
1 120 |
|
OM |
tr |
|
|
1;3 |
|
α-Campholenal |
1 122 |
|
1 487(F) |
OM |
|
tr |
|
2;3 |
|
trans-Pinocarveol |
1 135 |
|
1 646(F) |
OM |
|
tr |
|
2;3 |
|
trans-p-Menth-2-en-1-ol |
1 136 |
1 139 |
1 624(L) |
OM |
tr |
0,04 |
|
1;2;3 |
|
cis-Verbenol |
1 137 |
|
1 650(F) |
OM |
|
tr |
|
2;3 |
|
(E)-Epoxy-ocimene |
1 137 |
|
1 486(L) |
OM |
tr |
|
|
2;3 |
|
cis-p-Menth-1,8-diene-1-ol |
1 138 |
|
1 667(F) |
OM |
|
tr |
|
2;3 |
|
neo-allo-Ocimene |
1 140 |
1 144 |
|
M |
|
0,06 |
|
1;3 |
|
trans-Verbenol |
1 140 |
|
1 672(F) |
OM |
|
tr |
|
2;3 |
|
Veratrol |
1 141 |
|
1 726(F) |
OM |
|
tr |
|
2;3 |
|
p-Menth-3-en-8-ol |
1 145 |
1 146 |
|
OM |
|
tr |
|
1;3 |
|
Citronellal |
1 148 |
1 158 |
|
OM |
|
0,01 |
|
1;3 |
|
(E)-Non-2-enal |
1 157 |
1 159 |
1 531(L,F,T) |
A |
0,51 |
0,01 |
0,24 |
1;2;3 |
|
Pinocarvone |
1 160 |
1 166 |
1 555(F) |
OM |
|
0,01 |
|
1;2;3 |
|
1,3-Dimetoxybenzene |
1 165 |
1 167 |
|
B |
|
|
0,05 |
1;3 |
|
Ethyl benzoate |
1 169 |
1 170 |
1 658(F) |
B |
|
0,09 |
|
1;2;3 |
|
Nonan-1-ol |
1 172 |
1 171 |
|
A |
|
|
0,05 |
1;3 |
|
Terpinen-4-ol |
1 174 |
1 178 |
1 597(L,F) |
OM |
0,37 |
0,27 |
0,05 |
1;2;3;4 |
|
Naphthalene |
1 178 |
1 183 |
1 721(L) |
B |
0,43 |
|
|
1;2;3 |
|
m-Cymen-8-ol |
1 176 |
|
1 846(F) |
OM |
|
tr |
|
2;3 |
|
p-Cymen-8-ol |
1 179 |
|
1 846(L,F) |
OM |
tr |
tr |
|
2;3 |
|
Cryptone |
1 183 |
1 185 |
|
IT |
0,06 |
tr |
|
1;3 |
|
Methyl salicylate |
1 190 |
1 191 |
1 760(L,F) |
B |
0,29 |
0,37 |
0,16 |
1;2;3 |
|
α-Terpineol |
1 192 |
1 195 |
1 693(F) |
OM |
0,16 |
0,15 |
0,07 |
1;2;3 |
|
Myrtenal |
1 195 |
|
1 614(F) |
OM |
|
tr |
|
2;3 |
|
trans-p-Menthan-2-one |
1 199 |
1 198 |
|
OM |
|
0,15 |
|
1;3 |
|
Decanal |
1 201 |
1 206 |
1 497(T) |
A |
0,04 |
tr |
0,61 |
1;2;3 |
|
Verbenone |
1 204 |
|
1 688(F) |
OM |
|
tr |
|
2;3 |
|
trans-Piperitol |
1 207 |
1 209 |
|
OM |
0,02 |
0,07 |
|
1;3 |
|
Octyl acetate |
1 211 |
1 214 |
|
A |
0,07 |
|
|
1;3 |
|
trans-Carveol |
1 215 |
1 219 |
1 831(F) |
OM |
|
tr |
|
1;2;3 |
|
(E,E)-2,4-Nonadienal |
1 220 |
1 221 |
|
A |
0,06 |
tr |
|
1;3 |
|
1-p-Menth-9-al |
1 221 |
1 222 |
|
OM |
|
|
tr |
1;3 |
|
β-Cyclocitral |
1 225 |
1 222 |
|
IT |
tr |
|
|
1;3 |
|
cis-Carveol |
1 226 |
|
1 861(F) |
OM |
|
tr |
|
2;3 |
|
Nerol |
1 227 |
1 230 |
|
OM |
0,11 |
0,02 |
tr |
1;3 |
|
Cumin aldehyde |
1 238 |
1 237 |
|
OM |
|
0,01 |
|
1;3 |
|
Carvone |
1 239 |
|
1 719(F) |
OM |
|
tr |
|
2;3 |
|
(Z)-Dec-3-en-1-ol |
1 242 |
1 245 |
|
A |
0,04 |
0,02 |
0,05 |
1;3 |
|
Geraniol |
1 249 |
1 248 |
|
OM |
0,06 |
0,04 |
0,06 |
1;3;4 |
|
(E)-Dec-4-en-1-ol |
1 259 |
1 252 |
|
A |
|
0,03 |
|
1;3 |
|
Pent-4-enyl hexanoate |
1 260 |
1 254 |
1 534(F) |
A |
|
tr |
|
1;2;3 |
|
(E)-Dec-2-enal |
1 260 |
1 261 |
1 638(L,T) |
A |
0,19 |
0,07 |
0,41 |
1;2;3 |
|
trans-Ascaridole glycol |
1 266 |
|
2 086(F) |
OM |
|
tr |
|
2;3 |
|
Ethyl salicylate |
1 266 |
1 261 |
1 796(L) |
B |
tr |
0,06 |
|
1;2;3 |
|
Nonanoic acid |
1 267 |
1 266 |
|
A |
|
|
0,05 |
1;3 |
|
Dodecanol |
1 271 |
1 271 |
|
A |
|
|
0,50 |
1;3 |
|
Dihydro-linalool acetate |
1 272 |
1 269 |
|
OM |
|
tr |
|
1;3 |
|
p-Menth-1-en-7-al (Phellandral) |
1 280 |
1 283 |
|
OM |
0,02 |
0,12 |
|
1;3 |
|
Car-2-en-10-al |
1 289 |
1 281 |
|
OM |
|
tr |
|
1;3 |
|
p-Cymen-7-ol (Cumic alcohol) |
1 289 |
|
2 093(F) |
OM |
|
tr |
|
2;3 |
|
(2Z,4Z)-Deca-2,4-dienal |
1 292 |
1 292 |
|
A |
tr |
|
0,05 |
1;3 |
|
Undecan-2-one |
1 293 |
1 293 |
1 598(T) |
A |
|
|
0,05 |
1;2;3 |
|
Carvacrol |
1 298 |
1 296 |
|
OM |
0,05 |
|
|
1;3 |
|
2-Methylnaphthalene |
1 298 |
1 299 |
1 830(L) |
B |
0,06 |
|
|
1;2;3 |
|
Undecanal |
1 305 |
1 306 |
|
A |
|
|
0,02 |
1;3 |
|
4-Hydroxy-cryptone |
1 314 |
|
2 238(F) |
IT |
|
tr |
|
2;3 |
|
(2E,4E)-Deca-2,4-dienal |
1 315 |
1 317 |
|
A |
0,10 |
0,05 |
0,27 |
1;3 |
|
Myrtenyl acetate |
1 324 |
1 324 |
|
OM |
|
tr |
|
1;3 |
|
cis-Sabinyl acetate |
1 325 |
1 336 |
|
OM |
|
0,01 |
|
1;3 |
|
α-Cubebene |
1 345 |
1 351 |
1 453(F,T) |
S |
2,10 |
2,14 |
tr |
1;2;3 |
|
(E)-Undec-2-enal |
1 357 |
1 363 |
|
A |
|
|
0,20 |
1;3 |
|
Neryl acetate |
1 359 |
1 364 |
|
OM |
0,04 |
|
|
1;3 |
|
Cyclosativene |
1 369 |
1 366 |
|
S |
tr |
|
|
1;3 |
|
trans-p-menth-6-en-2,8-diol |
1 371 |
|
2 314(F) |
OM |
|
tr |
|
2;3 |
|
α-Ylangene |
1 373 |
1 373 |
1 473(L,F,T) |
S |
0,07 |
0,06 |
0,11 |
1;2;3 |
|
Isoledene |
1 374 |
1 374 |
|
S |
|
0,04 |
|
1;3 |
|
α-Copaene |
1 374 |
1 378 |
1 484(L,F,T) |
S |
2,22 |
2,24 |
2,19 |
1;2;3 |
|
Geranyl acetate |
1 379 |
1 382 |
|
OM |
|
0,02 |
|
1;3 |
|
β-Cubebene |
1 387 |
1 385 |
1 529(L) |
S |
tr |
0,20 |
|
1;2;3 |
|
β-Bourbonene |
1 387 |
1 387 |
1 508(L,F,T) |
S |
0,28 |
0,14 |
0,32 |
1;2;3 |
|
α-Bourbonene |
1 388 |
1 388 |
1 501(L) |
S |
0,10 |
|
|
1;2;3 |
|
β-Elemene |
1 389 |
1 393 |
1 582(F) |
S |
0,38 |
0,41 |
0,18 |
1;2;3 |
|
Dec-9-enyl acetate |
1 399 |
1 398 |
|
A |
0,08 |
0,10 |
|
1;3 |
|
Tetradecane |
1 400 |
1 400 |
1 400(T) |
A |
|
|
tr |
1;2;3 |
|
(Z)-Caryophyllene |
1 408 |
1 407 |
1 563(F) |
S |
|
tr |
|
1;2;3 |
|
α-Gurjunene |
1 409 |
1 412 |
1 517(L,F,T) |
S |
0,88 |
0,88 |
0,76 |
1;2;3 |
|
(E)-Caryophyllene |
1 417 |
1 422 |
1 584(L,F,T) |
S |
5,16 |
0,70 |
4,68 |
1;2;3;4 |
|
(E)-α-Ionone |
1 428 |
1 431 |
1 838(L) |
IT |
0,13 |
|
|
1;2;3 |
|
β-Copaene |
1 430 |
|
1 578(L,F) |
S |
tr |
tr |
|
2;3 |
|
α-Muurolene |
1 431 |
1 432 |
|
S |
|
|
0,21 |
1;3 |
|
γ-Elemene |
1 434 |
1 435 |
|
S |
0,22 |
0,47 |
0,21 |
1;3 |
|
cis-Thujopsene |
1 435 |
1 436 |
|
S |
|
|
0,05 |
1;3 |
|
α-Guaiene |
1 437 |
1 438 |
|
S |
0,08 |
0,07 |
0,10 |
1;3 |
|
Aromadendrene |
1 439 |
|
1 629(L,F,T) |
S |
tr |
tr |
tr |
2;3 |
|
6,9-Guaiadiene |
1 442 |
1 441 |
|
S |
|
0,11 |
|
1;3 |
|
cis-Muurola-3,5-diene |
1 448 |
1 444 |
|
S |
|
|
0,42 |
1;3 |
|
trans-Muurola-3,5-diene |
1 451 |
1 450 |
1 616(T) |
S |
|
0,05 |
tr |
1;2;3 |
|
α-Humulene |
1 452 |
1 456 |
1 674(L,F,T) |
S |
2,80 |
3,55 |
2,38 |
1;2;3;4 |
|
Geranyl acetone |
1 453 |
1 457 |
1 857(L) |
IT |
|
|
0,16 |
1;2;3 |
|
Allo-aromadendrene |
1 458 |
1 463 |
|
S |
0,48 |
0,47 |
0,59 |
1;3 |
|
cis-cadina-1(16),4-diene |
1 461 |
1 464 |
|
S |
tr |
|
0,76 |
1;3 |
|
cis-Muurola-4(14),5-diene |
1 465 |
1 464 |
|
S |
|
tr |
|
1;3 |
|
Cabreuva oxide C |
1 466 |
|
1 737(L) |
OS |
tr |
|
|
2;3 |
|
trans-cadina-1(16),4-diene |
1 475 |
1 475 |
1 648(L,T) |
S |
0,48 |
0,97 |
tr |
1;2;3 |
|
γ-Muurolene |
1 478 |
1 478 |
1 675(L,F) |
S |
0,21 |
0,10 |
0,31 |
1;2;3 |
|
α-Amorphene |
1 483 |
1 484 |
|
S |
|
0,12 |
|
1;3 |
|
Germacrene D |
1 484 |
1 483 |
1 693(L) |
S |
0,80 |
|
0,75 |
1;2;3 |
|
(E)-β-Ionone |
1 487 |
|
1 922(L) |
IT |
tr |
|
|
2;3 |
|
β-Selinene |
1 489 |
1 489 |
1 700(L,F) |
S |
tr |
tr |
0,31 |
1;2;3 |
|
trans-Muurola-4(14),5-diene |
1 493 |
1 494 |
1 695(L) |
S |
0,16 |
0,68 |
0,33 |
1;2;3 |
|
epi-Cubebol |
1 493 |
|
1 927(L) |
OS |
tr |
|
|
2;3 |
|
Valencene |
1 496 |
|
1 718(L) |
S |
tr |
|
|
2;3 |
|
Viridiflorene |
1 496 |
|
1 680(L) |
S |
tr |
0,31 |
|
2;3 |
|
α-Selinene |
1 498 |
1 497 |
1 706(L) |
S |
0,32 |
|
0,32 |
1;2;3 |
|
Pseudowiddrene |
1 498 |
|
1 661(L,F) |
S |
tr |
tr |
|
2;3 |
|
10,11-Epoxycalamenene |
1 498 |
|
1 868(F) |
OS |
|
tr |
|
2;3 |
|
α-Muurolene |
1 500 |
1 500 |
1 712(L,T) |
S |
0,54 |
|
0,81 |
1;2;3 |
|
Cuparene |
1 504 |
1 502 |
|
S |
|
|
0,05 |
1;3 |
|
β-Bisabolene |
1 505 |
1 504 |
|
S |
|
|
tr |
1;3 |
|
Germacrene A |
1 508 |
1 508 |
|
S |
0,43 |
0,18 |
0,17 |
1;3 |
|
α-Bulnesene |
1 509 |
1 506 |
|
S |
tr |
|
|
1;3 |
|
(E,E)-α-Farnesene |
1 509 |
1 509 |
|
S |
tr |
|
|
1;3 |
|
γ-Cadinene |
1 513 |
1 516 |
1 748(F,T) |
S |
0,17 |
tr |
0,14 |
1;2;3 |
|
Cubebol |
1 514 |
1 515 |
|
OS |
|
0,18 |
|
1;3 |
|
Geranyl isobutanoate |
1 514 |
|
1 857(L,F) |
M |
tr |
tr |
|
2;3 |
|
α-dehydro-ar-Himachalene |
1 516 |
|
1 888(F,T) |
S |
|
tr |
tr |
2;3 |
|
7-epi-α-Selinene |
1 520 |
|
1 760(L) |
S |
tr |
|
|
2;3 |
|
trans-Calamenene |
1 521 |
|
1 820(F, T) |
S |
|
tr |
tr |
2;3 |
|
δ-Cadinene |
1 522 |
1 524 |
1 742(L,T) |
S |
tr |
|
3,28 |
1;2;3;4 |
|
cis-Calamenene |
1 528 |
1 527 |
1 822(L,F) |
S |
12,60 |
12,70 |
11,31 |
1;2;3 |
|
α-dehydro-ar-himachalene |
1 530 |
|
1 887(L) |
S |
tr |
|
|
2;3 |
|
trans-Cadina-1,4-diene |
1 533 |
1 535 |
1 766(L,T) |
S |
1,00 |
1,38 |
1,40 |
1;2;3 |
|
10-epi-Cubebol |
1 533 |
|
1 875(L) |
S |
tr |
|
|
2;3 |
|
α-Cadinene |
1 537 |
1 538 |
1 776(L) |
S |
0,16 |
0,33 |
|
1;2;3 |
|
α-Calacorene |
1 544 |
1 545 |
1 896(L,T) |
S |
0,40 |
0,41 |
0,63 |
1;2;3 |
|
cis-Muurolol-5-en-4-β-ol |
1 550 |
|
1 876(F) |
OS |
|
tr |
|
2;3 |
|
Germacrene B |
1 559 |
1 560 |
1 806(L,T) |
S |
1,94 |
3,65 |
0,07 |
1;2;3 |
|
(E)-Nerolidol |
1 561 |
1 565 |
2 041(L,T) |
OS |
0,59 |
|
0,57 |
1;2;3;4 |
|
β-Calacorene |
1 564 |
|
1 939(L,F) |
S |
tr |
tr |
|
2;3 |
|
Dodecanoic acid |
1 565 |
1 566 |
|
A |
|
|
0,24 |
1;3 |
|
Palustrol |
1 567 |
1 570 |
|
OS |
|
tr |
|
1;3 |
|
Spathulenol |
1 577 |
|
2 107(L,F,T) |
OS |
tr |
0,10 |
tr |
2;3 |
|
(Z)-Caryophyllene oxide |
1 580 |
1 578 |
1 949(L,F,T) |
OS |
tr |
tr |
tr |
1;2;3 |
|
(E)-Caryophyllene oxide |
1 582 |
1 586 |
1 956(L) |
OS |
2,04 |
2,93 |
2,94 |
1;2;3 |
|
Gleenol |
1 586 |
1 587 |
2 024(L,F,T) |
OS |
tr |
0,27 |
0,50 |
1;2;3 |
|
Globulol |
1 590 |
|
2 056(F) |
OS |
|
tr |
|
2;3 |
|
Viridiflorol |
1 592 |
|
2 062(L) |
OS |
tr |
|
|
2;3 |
|
Hexadecane |
1 600 |
|
1 600(L,T) |
A |
tr |
|
tr |
2;3 |
|
Ledol (epi-Globulol) |
1 602 |
1 607 |
2 005(L,F,T) |
OS |
0,58 |
0,28 |
0,59 |
1;2;3 |
|
Humulene epoxide II |
1 608 |
1 609 |
2 012(L,F,T) |
OS |
0,92 |
0,48 |
0,93 |
1;2;3 |
|
1,10-di-epi-cubenol |
1 618 |
|
2 038(L,T) |
OS |
tr |
tr |
tr |
2;3 |
|
Junenol |
1 618 |
1 620 |
2 031(L,T) |
OS |
0,07 |
0,85 |
0,17 |
1;2;3 |
|
1-epi-Cubenol |
1 627 |
1 632 |
2 046(L,F,T) |
OS |
1,87 |
1,41 |
2,45 |
1;2;3 |
|
Muurola-4,10(14)-dien-1β-ol |
1 630 |
|
2 132(L) |
OS |
tr |
|
|
2;3 |
|
epi-α-Cadinol (tau-Cadinol) |
1 638 |
|
2 156(L,T) |
OS |
tr |
tr |
tr |
2;3 |
|
Caryophylla-4(12),8(13)-dien-5α-ol |
1 639 |
1 638 |
2 278(F) |
OS |
tr |
0,16 |
0,48 |
1;2;3 |
|
Caryophylla-4(12),8(13)-dien-5β-ol |
1 639 |
1 641 |
2 272(F) |
OS |
|
0,17 |
|
1;2;3 |
|
Hinesol |
1 640 |
1 642 |
|
OS |
|
2,16 |
|
1;3 |
|
epi-α-Muurolol |
1 640 |
1 643 |
2 172(L,F,T) |
OS |
tr |
0,50 |
tr |
1;2;3 |
|
α-Muurolol |
1 644 |
1 645 |
2 186(L,F) |
OS |
tr |
0,65 |
|
1;2;3 |
|
Cubenol |
1 645 |
1 646 |
|
OS |
2,45 |
|
3,24 |
1;3 |
|
α-Cadinol |
1 652 |
1 658 |
2 216(L,F,T) |
OS |
1,42 |
1,18 |
0,62 |
1;2;3 |
|
Selin-11-en-4-α-ol |
1 658 |
1 659 |
2 231(L,F,T) |
OS |
0,05 |
tr |
tr |
1;2;3 |
|
cis-Calamenen-10-ol |
1 660 |
|
2 323(L,F) |
OS |
tr |
tr |
|
2;3 |
|
trans-Calamenen-10-ol |
1 668 |
1 666 |
2 353(L,F) |
OS |
tr |
0,05 |
|
1;2;3 |
|
Tetradecanol |
1 671 |
1 676 |
|
A |
|
1,14 |
|
1;3 |
|
Cadalene |
1 675 |
1 678 |
2 198(L,F) |
S |
0,08 |
tr |
0,62 |
1;2;3 |
|
Mustakone |
1 676 |
|
2 223(F) |
IT |
|
tr |
|
2;3 |
|
Muurola-4,10(14)-dien-1-β-ol |
1 686 |
1 683 |
|
OS |
|
|
0,46 |
1;3 |
|
Eudesma-4(15),7-dien-1β-ol |
1 687 |
1 683 |
|
OS |
0,25 |
0,31 |
|
1;3 |
|
Pentadecan-2-one |
1 697 |
|
2 120(T) |
A |
|
|
tr |
2;3 |
|
Eudesma-7(11)-en-4-ol (Juniper camphor) |
1 694 |
1 690 |
|
OS |
tr |
0,30 |
0,18 |
1;3 |
|
Heptadecane |
1 700 |
1 700 |
|
A |
|
|
0,09 |
1;3 |
|
Amorpha-4,9-dien-2-ol |
1 700 |
1 699 |
2 336(L,F) |
OS |
0,17 |
tr |
|
1;2;3 |
|
10-nor-Calamenen-10-one |
1 702 |
|
2 349(F) |
OS |
|
tr |
|
2;3 |
|
5-Hydroxy-cis-calamenene |
1 713 |
|
2 325(F) |
OS |
|
tr |
|
2;3 |
|
(2E,6Z)-Farnesol |
1 714 |
1 712 |
|
OS |
0,10 |
|
0,48 |
1;3 |
|
Nootkatol |
1 714 |
1 717 |
2 458(L) |
OS |
0,05 |
0,30 |
|
1;2;3 |
|
Pentadecanal |
1 717 |
1 717 |
|
A |
|
0,05 |
|
1;3 |
|
(2Z,6E)-Farnesol |
1 722 |
|
2 352(T) |
OS |
|
|
tr |
2;3 |
|
Benzyl benzoate |
1 759 |
1 766 |
2 603(L,F) |
B |
0,14 |
0,47 |
|
1;2;3 |
|
Tetradecanoic acid |
1 762 |
1 767 |
2 722(T) |
A |
|
|
0,86 |
1;2;3 |
|
14-Hydroxy-α-muurolene |
1 779 |
1 773 |
|
OS |
|
tr |
|
1;3 |
|
14-Hydroxy-δ-cadinene |
1 803 |
1 806 |
|
OS |
0,05 |
0,05 |
|
1;3 |
|
Hexadecanal |
1 819 |
1 822 |
|
A |
0,01 |
0,05 |
0,16 |
1;3 |
|
Hexahydrofarnesyl acetone |
1 843 |
1 846 |
2 125(L) |
IT |
0,05 |
tr |
0,30 |
1;2;3 |
|
Pentadecanoic acid |
1 857 |
1 858 |
|
A |
|
|
0,11 |
1;3 |
|
Benzyl salicylate |
1 864 |
1 870 |
2 751(L) |
B |
0,05 |
|
|
1;2;3 |
|
Hexadecan-1-ol |
1 874 |
1 879 |
2 378(T) |
A |
0,01 |
tr |
0,12 |
1;2;3 |
|
Nonadec-1-ene |
1 895 |
1 894 |
|
A |
|
|
tr |
1;3 |
|
Nonadecane |
1 900 |
1 900 |
1 900(T) |
A |
|
|
0,40 |
1;2;3 |
|
Heptadecan-2-one |
1 908 |
1 908 |
|
A |
|
tr |
|
1;3 |
|
(5E,9E)-Farnesyl acetone |
1 913 |
1 906 |
|
IT |
|
|
0,06 |
1;3 |
|
Heptadecanal |
1 920 |
1 916 |
|
A |
|
|
tr |
1;3 |
|
Methyl hexadecanoate |
1 921 |
1 923 |
|
A |
|
|
tr |
1;3 |
|
Isophytol |
1 946 |
1 947 |
|
D |
|
tr |
|
1;3 |
|
(Z)-Hexadec-9-enoic acid |
1 952 |
1 949 |
|
A |
|
|
0,20 |
1;3 |
|
Geranyl benzoate |
1 958 |
1 960 |
|
M |
0,02 |
tr |
|
1;3 |
|
Hexadecanoic acid (Palmitic acid) |
1 959 |
1 961 |
2 932(L,F,T) |
A |
tr |
0,06 |
7,99 |
1;2;3;4 |
|
Ethyl hexadecanoate |
1 993 |
1 990 |
2 250(T) |
A |
|
|
0,39 |
1;2;3 |
|
Eicosane |
2 000 |
2 000 |
|
A |
|
|
0,06 |
1;3 |
|
Manool oxide |
2 009 |
2 002 |
|
D |
|
|
0,16 |
1;3 |
|
Hexadecan-1-ol acetate |
2 010 |
2 009 |
|
A |
|
tr |
|
1;3 |
|
(E,E)-Geranyl linalool |
2 026 |
2 031 |
2 535(L,T) |
D |
0,05 |
tr |
0,28 |
1;2;3 |
|
Heneicosane |
2 100 |
2 100 |
2 100(T) |
A |
|
|
0,66 |
1;2;3 |
|
Nonadecan-2-one |
2 101 |
2 100 |
|
A |
|
tr |
|
1;3 |
|
(E)-Phytol |
2 107 |
|
2 612(L,F,T) |
D |
tr |
tr |
1,20 |
2;3 |
|
(Z)-Phytol |
2 114 |
2 113 |
2 412(L) |
D |
0,13 |
0,05 |
|
1;2;3 |
|
Linoleic acid |
2 134 |
2 132 |
|
A |
|
0,08 |
0,74 |
1;3 |
|
Oleic acid |
2 141 |
2 135 |
|
A |
|
|
0,63 |
1;3 |
|
Palmitaldehyde, diallyl acetal |
2 148 |
2 147 |
|
A |
tr |
tr |
|
1;3 |
|
Ethyl linoleate |
2 155 |
2 162 |
2 518(T) |
A |
|
tr |
0,43 |
1;2;3 |
|
Ethyl linolenate |
2 169 |
2 169 |
|
A |
|
tr |
|
1;3 |
|
Nonadecan-1-ol |
2 181 |
2 188 |
|
A |
|
|
0,06 |
1;3 |
|
Docosane |
2 200 |
2 200 |
2 200(T) |
A |
|
|
0,25 |
1;2;3;4 |
|
Eicosanal |
2 219 |
2 220 |
|
A |
|
|
tr |
1;3 |
|
Tricosane |
2 300 |
2 300 |
2 300(T) |
A |
|
|
0,53 |
1;2;3;4 |
|
Heneicosan-2-one |
2 306 |
2 305 |
|
A |
|
|
0,08 |
1;3 |
|
Tetracosane |
2 400 |
2 400 |
2 400(T) |
A |
|
|
0,33 |
1;2;3;4 |
|
Pentacosane |
2 500 |
2 500 |
2 500(T) |
A |
|
|
0,70 |
1;2;3;4 |
|
Hexacosane |
2 600 |
2 600 |
2 600(T) |
A |
|
|
0,19 |
1;2;3;4 |
|
Heptacosane |
2 700 |
2 700 |
2 700(T) |
A |
|
|
0,07 |
1;2;3;4 |
|
Octacosane |
2 800 |
2 800 |
2 800(T) |
A |
|
|
tr |
1;2;3;4 |
|
Nonacosane |
2 900 |
2 900 |
2 900(T) |
A |
|
|
tr |
1;2;3;4 |
|
TOTAL |
|
|
|
|
91,29 |
86,65 |
74,56 |
|
|
No. of compounds |
|
|
|
|
160 |
199 |
144 |
|
|
|
|
|
|
|
|
|
|
|
|
Compound class |
|
|
|
|
|
|
|
|
|
Total monoterpenoids |
|
|
|
|
42,66 |
38,63 |
6,84 |
|
|
Monoterpene hydrocarbons (M) |
|
|
|
|
36,98 |
30,75 |
6,20 |
|
|
Oxygenated monoterpenes (OM) |
|
|
|
|
5,68 |
7,88 |
0,64 |
|
|
Total sesquiterpenoids |
|
|
|
|
44,67 |
44,69 |
46,45 |
|
|
Sesquiterpene hydrocarbons (S) |
|
|
|
|
34,06 |
32,36 |
32,84 |
|
|
Oxygenated sesquiterpenes (OS) |
|
|
|
|
10,61 |
12,33 |
13,61 |
|
|
Diterpenoids (D) |
|
|
|
|
0,18 |
0,05 |
1,64 |
|
|
Irregular terpenoids (IT) |
|
|
|
|
0,24 |
tr |
0,52 |
|
|
Aliphatics (A) |
|
|
|
|
2,55 |
2,17 |
18,77 |
|
|
Benzenoids (B) |
|
|
|
|
0,91 |
1,01 |
0,29 |
|
|
Miscellaneous (Misc.) |
|
|
|
|
tr |
0,1 |
0,05 |
|
aCompounds listed in order of elution from poly-(5% phenyl 95% dimethylsiloxane) type column. bRILit = DB-5 (Adams, 2007; Wallace, 2021). cRIExp = Retention index relative to C8-C32 n-alkanes on the SLB™-5ms column. dSw10Exp = Experimental retention index on Supelcowax™ 10. eIM = Identification methods: 1 = Retention index on poly-(5% phenyl/95% methylsiloxane) type column; 2 = Retention index on Supelcowax™10; 3 = MS spectra; 4 = Standard. ftr = Traces (<0,005%). *(Romanenko & Tkachev, 2006; Collin et al., 2010). Major terpenoids are in boldface.