
SHORT COMMUNICATION
Bacterial multi-resistance to antibiotics in water from plant cavities (phytotelms) in a deep tropical forest from Costa Rica
Stephanny
Sánchez-Vargas1![]() , Paula Vargas-Jiménez2
, Paula Vargas-Jiménez2![]() , Luis
Vega-Corrales1
, Luis
Vega-Corrales1![]() & Junior
Pastor Pérez-Molina2
& Junior
Pastor Pérez-Molina2![]()
1. Universidad Nacional, Escuela de Ciencias Biológicas, Laboratorio de Microbiología Marina (LaMMaR), Heredia, Costa Rica; stephanny.sanchez.vargas@est.una.ac.cr, luis.vega.corrales@una.cr
2. Universidad Nacional, Escuela de Ciencias Biológicas, Laboratorio de Ecología Funcional y Ecosistemas Tropicales (LEFET), Heredia, Costa Rica; paula.vargas.jimenez@est.una.ac.cr, junior.perez.molina@una.cr
Received 08-III-2023 ● Corrected 14-VII-2023 ● Accepted 20-VII-2023
DOI: https://doi.org/10.22458/urj.v15i2.4675
|
ABSTRACT. Introduction: The spread of antimicrobial resistance in natural environments continues to be reported throughout the world; nevertheless, there is no study about phytotelms (water in the natural cavities of plants) in the deep understory of tropical cloud forests from Costa Rica. Objective: To detect phytotelm antibiotic-resistant bacterial strains in a Costa Rican forest and nearby town. Methods: We used the disk diffusion method to analyze the antimicrobial resistance of 10 gram-negative bacterial strains from phytotelm water collected from 10 bromeliads and 10 heliconias in both sites. Results: Three strains were multidrug resistant to more than three antibiotics in each site, and only one strain was susceptible to all antibiotics. Antibiotic resistance was similar in both environments. Conclusions: Phytotelm can be a rapid, cost-effective, and simple source for detecting antimicrobial resistance in unexplored environments.
Keywords: Monteverde cloud forest, urban zone, antimicrobial resistance (AMR), bromeliads tank, understory, deep forest.
|
RESUMEN. “Multirresistencia bacteriana a los antibióticos en el agua de cavidades vegetales (fitotelmas) de un bosque tropical profundo de Costa Rica”. Introducción: La propagación de la resistencia antimicrobiana en entornos naturales continúa siendo informada de todo el mundo; sin embargo, no existe ningún estudio sobre fitotelmias (agua en las cavidades naturales de las plantas) en el sotobosque profundo de los bosques nubosos tropicales de Costa Rica. Objetivo: Detectar cepas bacterianas resistentes a antibióticos en fitotelmas de un bosque costarricense y un pueblo cercano. Métodos: Utilizamos el método de difusión en disco para analizar la resistencia antimicrobiana de 10 cepas bacterianas gram-negativas aisladas del agua de fitotelmas de 10 bromelias y 10 heliconias, en ambos sitios. Resultados: Tres cepas fueron resistentes a más de tres antibióticos en cada sitio, y una fue susceptible a todos los antibióticos. La resistencia a los antibióticos fue similar en ambos entornos. Conclusiones: Las fitotelmas pueden ser una fuente rápida, económica y sencilla para detectar la resistencia antimicrobiana en entornos inexplorados.
Palabras clave: bosque nuboso de Monteverde, zona urbana, resistencia antimicrobiana (RAM), tanque de bromelias, sotobosque, bosque profundo. |
A better understanding of the generation of antimicrobial resistance (AMR) and its presence in poorly investigated natural environments can lead to combating this global health challenge. AMR is constantly increasing due to the mismanagement of antimicrobial compounds (Lee & Lin, 2003) and continues to be reported worldwide, expanding into natural environments as a result of urbanization and vector spread. In fact, chloramphenicol-resistant strains have even been detected in surface waters of isolated cave microbiomes in Mexico (Bhullar et al., 2012).
Water catchments stored in the natural cavities of plants (phytotelms), such as bromeliad tanks, are commonly used as bioindicators. Their physicochemical and geochemical characteristics, as well as the communities of macro- and micro-organisms, and their interactions, make them suitable matrices for evaluating ecological interactions, carbon and nitrogen cycles, and environmental stresses (Benavides-Gordillo et al., 2019). Moreover, the bacterial communities within bromeliad tanks are unique, and their bacterial abundance remains stable even during drought or increased rainfall events (Rodríguez-Pérez et al., 2018; Benavides-Gordillo et al., 2019).
To date, no studies have investigated AMR in phytotelm samples from a tropical cloud forest in Costa Rica. The closest relevant research was conducted by Brighigna et al. (1997), who successfully used Tillandsia caput-medusae (Bromeliaceae) as a biomonitor of air pollution by lead, copper, and cadmium. Therefore, the aim of this research was to assess the potential of phytotelms as a matrix for detecting antibiotic-resistant bacterial strains in the deep understory of a tropical cloud forest and a nearby rural town in Costa Rica. We employed a simple, inexpensive, and rapid method to evaluate these bacterial communities and determine the extent to which antibiotics have penetrated the forest before proceeding with molecular identification. Additionally, we conducted a text mining analysis to investigate the current information available on "antibiotic resistance in forests" and determine the direction of existing studies. We isolated six gram-negative bacterial strains from the forest and four from the urban site. Only one strain was susceptible to all antibiotics and three strains were multi-resistant to different antibiotics (>3) in each site (Fig. 1A, B). Congruently, NMDS and PERMANOVA revealed that both locations had a similar response to antibiotic resistance (F(1,13) = 1,02, P>0,05, Fig. 1C).
We identified 71 articles that aligned with our search conducted on PubMed.gov. Among this limited number of articles, there has been a significant and rapid increase in interest in studying the presence of antibiotic resistance in forests over the past five years (Figure 2A). The oldest was an article on the ecology of aquatic bacteria and multiple antibiotic resistance (Ogan & Nwiika, 1993), but it was not until the years 2018-2021 that the interest in this topic experienced a notable upturn. Figure 2B illustrates the recurring concepts associated with forests, including genes, soil microbiology, environmental monitoring, agriculture, land use, rivers, antibiotic resistance genes, and microbiota. We raise the hypothesis that the AMR could be introduced into the forest by the exposition of antimicrobial resistance genes (ARGs), resistant bacteria, or antibiotic molecules transported by different dissemination pathways such as wind, precipitation, air pollution, or as via fine particulate matter (PM 2.5) contained in the atmosphere (Xie et al., 2018; Zhu et al., 2021). Segawa et al. (2013), revealed the presence of resistance genes due to clinical tests in polar snow and glaciers, where humans have not intervened, and argue that these were spread by bacteria through the air. The phytotelm is a complex and small ecological habitat with several community interactions, where bacteria produce their antimicrobial compounds to compete for space and nutrients, leafding to the generation of natural resistance. In addition, bacteria can mutate, adapt, and transfer multi-resistance genes, which may enhance the mechanisms of AMR gene transfection in this small niche (von Wintersdorff et al., 2016).

Fig. 1. A) Test of gram-negative bacterial antibiotic resistance categorized by type antibiotic and inhibition mechanism for forest and urban sites. The size of the circles shows the diameter of the bacterial growth inhibition halo. Bacteria with three or more resistant antibiotics are designated as multi-resistant. B) Illustration of two antibiograms. C) Non-metric multi-dimensional scaling (NMDS, presence/absence) of bacterial antibiotic resistance in phytotelm for forest and urban sites. Antibiotic: AMC—Amoxicillin/Clavulanic Acid, AMP—Ampicillin, C—Chloramphenicol, CIP—Ciprofloxacin, CN—Gentamicin, CTX—Cefotaxime, ENR—Enrofloxacin, FFC—Florfenicol, FOS—Fosfomycin, OFX—Ofloxacin, OT—Oxytetracycline, SXT—Trimethoprim/Sulfamethoxazole, and TE—Tetracycline
This is the first study to attempt to prove the presence of bacterial strains with some degree of antibiotic resistance in phytotelms from the deep understory of a tropical cloud forest from Costa Rica. The potential dangers of the emergence of antibiotic resistance in natural ecosystems are uncertain. Efforts to determine the impact of antibiotic resistance on the human population estimated that deaths from antimicrobial resistance could rise from about 700,000 a year to about 10 million deaths per year by 2050 (O’Neill, 2014), despite criticism from Kraker et al., (2016) and NOAH (2016). Although antibiotic resistance is a natural process, the use of antimicrobials has increased significantly in recent decades, by exposing bacteria to greater numbers and increased concentration, which increases their chances of developing resistance.
Our results open the possibility of using the phytotelm as a convenient and practical matrix for detecting AMR dissemination. The bacterial communities within these water catchments exhibit remarkable stability and resistance to fluctuations in water volume. This unique characteristic makes them an ideal matrix for assessing long-term environmental changes, distinguishing them from other commonly used samples. Additionally, this approach enables simultaneous monitoring of large protected forest areas, offering a fast and efficient tool.
Further research is necessary to determine whether AMR is primarily influenced by anthropogenic factors or occurs through natural mechanisms of bacterial AMR generation. While bacterial antibiotic susceptibility tests using the disk diffusion technique are a cost-effective, easy, and rapid method for identifying resistant and multi-resistant bacteria, they do not address antibiotic resistance genes (ARGs) or resistance in non-cultivable bacteria. However, the detection of traces of antibiotic compounds in the phytotelm can complement this method.
Lastly, it is crucial to consider how ecological and physicochemical factors can influence AMR patterns in different geographical locations where samples are collected.
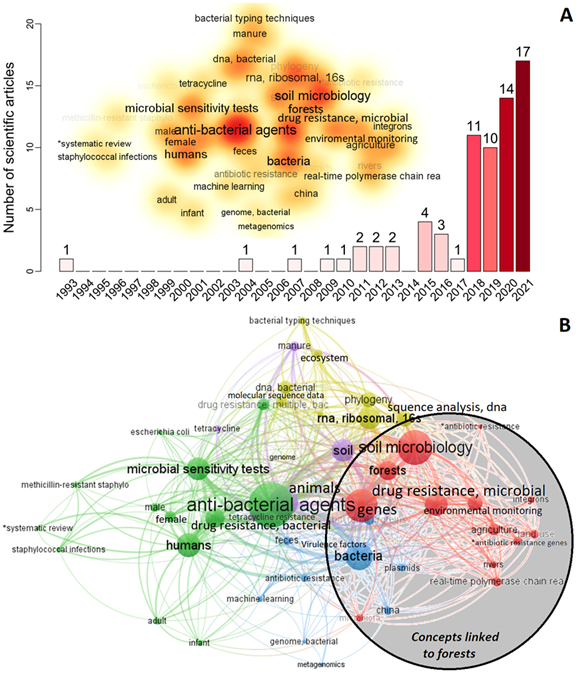
Fig. 2. A) The number of scientific articles about bacterial antibiotic resistance in forests, 1993—2020 (n = 71). Word cloud is the analysis of the frequency of the keywords of all the articles with co-occurrences greater than three words. B) Network analyses keywords of all the scientific articles with co-occurrences greater than three words
ACKNOWLEDGEMENTS
We thank to LEFET and LaMMaR of the Escuela de Ciencias Biológicas of the Universidad Nacional, Costa Rica, for allowing the development of this research. We also thank Roberto Cordero Solórzano and Stefany Solano González for editing the English grammar.
ETHICAL, CONFLICT OF INTEREST AND FINANCIAL STATEMENTS
The authors declare that they have fully complied with all pertinent ethical and legal requirements, both during the study and in the production of the manuscript; that there are no conflicts of interest of any kind; that all financial sources are fully and clearly stated in the acknowledgements section; and that they fully agree with the final edited version of the article. A signed document has been filed in the journal archives.
This work was supported by Funds-UNA-Costa Rica, within the framework of the project: Institutional Fund for Academic Development (FIDA; SIA 0597-19), LEFET (SIA 0156-18), and LaMMaR (SIA 0025-20).
The statement of each author’s contribution to the manuscript is as follows: S.S-V: bacterial isolation, microbiological analysis, writing, and editing. P. V-J: conducted the fieldwork and bacterial isolation. LV-C: study design, microbiological analysis, writing and editing. JP.P-M: study design, conducted the fieldwork, statistical analysis, writing, and editing.
REFERENCES
Benavides‐Gordillo, S., Farjalla, V. F., González, A. L., & Romero, G. Q. (2019). Changes in rainfall level and litter stoichiometry affect aquatic community and ecosystem processes in bromeliad phytotelmata. Freshwater Biology, 64(8), 1357–1368. https://doi.org/10.1111/fwb.13310
Bhullar, K., Waglechner, N., Pawlowski, A., Koteva, K., Banks, E. D., Johnston, M. D., Barton, H. A., & Wright, G. D. (2012). Antibiotic resistance is prevalent in an isolated cave microbiome. PLOS One, 7(4), e34953. https://doi.org/10.1371/journal.pone.0034953
Brighigna, L., Ravanelli, M., Minelli, A., & Ercoli, L. (1997). The use of an epiphyte (Tillandsia caput-medusae morren) as bioindicator of air pollution in Costa Rica. The Science of the Total Environmental, 198(2), 175–180. https://doi.org/10.1016/S0048-9697(97)05447-8
Humphries, R., Bobenchik, A. M., Hindler, J. A., Schuetz, A. N. & McAdam, A. J. (2021). Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100, 31st Edition. Journal of Clinical Microbiology, 59(12), e00213–21. https://doi.org/10.1128/JCM.00213-21.
Kraker, M. E. A., Stewardson, A. J., & Harbarth, S. (2016). Will 10 million people die a year due to antimicrobial resistance by 2050? PLOS medicine, 13(11), e1002184. https://doi.org/10.1371/journal.pmed.1002184
Lee, P. R., & Lin, C. (2003). The antibiotic paradox: how the misuse of antibiotics destroys their curative powers (review). Perspectives in Biology and Medicine, 46(4), 603–604. https://doi.org/10.1353/pbm.2003.0088
NOAH. (2016). NOAH responds to the O’Neill review. Veterinary Record, 179(6), 132–132. https://doi.org/10.1136/vr.i4266
O’Neill, J. (2014). Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. https://goo.by/pUHMs
Ogan, M. T., & Nwiika, D. E. (1993). Studies on the ecology of aquatic bacteria of the lower Niger Delta: Multiple antibiotic resistance among the standard plate count organisms. The Journal of applied bacteriology, 74(5), 595–602.
Rodríguez-Pérez, H., Borrel, G., Leroy, C., Carrias, J.-F., Corbara, B., Srivastava, D. S., & Céréghino, R. (2018). Simulated drought regimes reveal community resilience and hydrological thresholds for altered decomposition. Oecologia, 187(1), 267–279. https://doi.org/10.1007/s00442-018-4123-5
Segawa, T., Takeuchi, N., Rivera, A., Yamada, A., Yoshimura, Y., Barcaza, G., Shinbori, K., Motoyama, H., Kohshima, S., & Ushida, K. (2013). Distribution of antibiotic resistance genes in glacier environments. Environmental Microbiology Reports, 5(1), 127–134. https://doi.org/10.1111/1758-2229.12011
von Wintersdorff, C. J. H., Penders, J., van Niekerk, J. M., Mills, N. D., Majumder, S., van Alphen, L. B., Savelkoul, P. H. M., & Wolffs, P. F. G. (2016). Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Frontiers in Microbiology, 7, 173. https://doi.org/10.3389/fmicb.2016.00173
Xie, J., Jin, L., Luo, X., Zhao, Z., & Li, X. (2018). Seasonal disparities in airborne bacteria and associated antibiotic resistance genes in PM2.5 between urban and rural sites. Environmental Science & Technology Letters, 5(2), 74–79. https://doi.org/10.1021/acs.estlett.7b00561
Zhu, G., Wang, X., Yang, T., Su, J., Qin, Y., Wang, S., Gillings, M., Wang, C., Ju, F., Lan, B., Liu, C., Li, H., Long, X.-E., Wang, X., Jetten, M. S. M., Wang, Z., & Zhu, Y.-G. (2021). Air pollution could drive global dissemination of antibiotic resistance genes. The ISME Journal, 15(1), 270-281. https://doi.org/10.1038/s41396-020-00780-2