SHORT COMMUNICATION
Rapid assessment of non-flying mammals in three levels of human disturbance, Hacienda Barú National Wildlife Refuge, Costa Rica
Diego
Fallas-Madrigal1,2,3![]() , Catherine Sanchez-González1
, Catherine Sanchez-González1![]() , Didier González-Mora1
, Didier González-Mora1![]() , Melissa Chavarría-Arroyo1
, Melissa Chavarría-Arroyo1![]() & Mercedes Penit-Llobet1
& Mercedes Penit-Llobet1![]()
1. Universidad Latina de Costa Rica, Escuela de Ciencias Biológicas, San José, Costa Rica; diegofallasmadrigal@gmail.com, cadasago18@gmail.com, tatog209@hotmail.com, melch071@hotmail.com, mpenitllobet@gmail.com
2. Universidad Nacional, Escuela de Ciencias Biológicas, Heredia, Costa Rica
3. Universidad de Costa Rica, Sistema de Estudios de Posgrado en Biología, San José, Costa Rica
Recibido 25-I-2023 □ Corregido 30-IV-2023 □ Aceptado 04-V-2023
DOI: https://doi.org/10.22458/urj.v15i1.4558
|
ABSTRACT. Introduction: Change of natural land use has become major a driver of biodiversity loss around the world. Mammals are important components of forests because they affect forest structure and composition, but few studies have compared mammals in tropical areas with different levels of human disturbance. Objective: To do a rapid assessment of non-flying mammals in Hacienda Barú National Wildlife Refuge, Costa Rica, in three zones with different levels of human disturbance. Methods: On July 18-21, 2019, we identified non-flying mammals with trail walk sightings, camera traps, and Sherman traps. Results: We identified 17 species but no differences among zones. The most common were Cebus imitador and Pecari tajacu, the most used plant was Mangifera indica. Conclusion: This brief study identified 17 non-flying mammals in this reserve.
Keywords: conservation, protection, first evaluation, forest, lowland |
RESUMEN. “Evaluación rápida de mamíferos no voladores en tres niveles de perturbación humana, Refugio Nacional de Vida Silvestre Hacienda Barú, Costa Rica”. Introducción: El cambio del uso natural de la tierra se ha convertido en uno de los principales impulsores de la pérdida de biodiversidad en todo el mundo. Los mamíferos son componentes importantes de los bosques porque afectan la estructura y composición del bosque, pero pocos estudios han comparado a los mamíferos en áreas tropicales con diferentes niveles de perturbación humana. Objetivo: Realizar una evaluación rápida de los mamíferos no voladores en el Refugio Nacional de Vida Silvestre Hacienda Barú, Costa Rica, en tres zonas con diferentes niveles de perturbación humana. Métodos: del 18 al 21 de julio de 2019, identificamos mamíferos no voladores con avistamiento en senderos, cámaras trampa y trampas Sherman. Resultados: Identificamos 17 especies, sin diferencias entre zonas. Las más comunes fueron Cebus imitador y Pecari tajacu, la planta más utilizada fue Mangifera indica. Conclusión: Este estudio identificó 17 mamíferos no voladores en esta reserva.
Palabras clave: conservación, protección, primer estudio, bosque, tierras bajas
|
Change of natural land use such as wetlands, native grasslands, forests, has become major driver of biodiversity loss around the world, altering species capacity to persist in these human-modified landscapes (Crandall et al., 2000; Smith et al., 2001; Laurance et al., 2014). This had led to habitat destruction, biological communitie’s modification, and species extinction (Newbold et al., 2015; Ceballos et al., 2017). Although specialists can disappear from fragmented landscapes, there are generalist species that can be favoured from it (Passamani & Fernandez, 2011).
Mammals are important components of forests, as well of key players of ecology restoration of secondary forests, since they affect its structure and composition by feeding on seeds and spreading them, making quickly changes, and eventually attracting more animal resettlement (Fedriani & Delibes, 2009; Andresen et al., 2018; Li et al. 2021). However, just a few studies have provided information about shifts in mammal species composition and diversity in non-protected or protected areas (Hagger et al., 2013; Bogoni et al., 2016). This can be a result of the intrinsic characteristics in mammal species that make some of them easier to find than others, such as body size, diurnality, habitat use and population density (Ladle et al., 2011). Also, extrinsic characteristics as geographical factors such as the overlap between distributions may play an important role for their study (Meyer et al., 2015).
Thus, one common strategy to counteract habitat loss and to promote species conservation protected areas are being created, which minimize the negative effects on biodiversity by letting species to mobilize through the landscape (Jules & Shahani, 2003; Dudley et al., 2010). Still, small reserves (~ 100ha) have been given less relevance (Volenec & Dobson 2020). However, recently the private sector has been increasingly recognized to play a substantial role in global biodiversity conservation (Stolton et al., 2014; UNEP-WCMC & IUCN, 2016). Thus, in order to contribute with the first report of mammals in the Hacienda Barú National Wildlife Refuge, here we propose a rapid assessment of non-flying mammals in the refuge, by determining their richness, abundance, structure, and interactions with the environment.
This study was carried out between the 18 and 21 of July of 2019 at the Hacienda Barú National Wildlife Refuge (from here on as Hacienda Barú), located in Puntarenas, Costa Rica. The refuge covers 330 hectares of protected area and is composed of primary and a secondary forest, approximately three kilometers of beach, plus another kilometer that borders the Barú river. With a mean temperature is 28,5°C and ~ 15m of maximum height, Hacienda Barú is part of a tropical wet forest based on the Holdridge life zones (Holdridge, 1978; The Weather Channel, 2023). We focused on the secondary forest and classified it in three different zones based on the characteristics and accessibility to the public. These were called Zone A: with access restricted to authorized personnel and dense forest; Zone B: with access to tourism and a mixture of dense forest with large grass patches; and Zone C: with access to tourists, human modified land use and forest with the vast majority in recovery. Each zone has walking trails established by Hacienda Barú (Fig. 1).
The trails of each zone were walked during the mornings, afternoons and nights of all days; with a moderated speed. Each mammal sighted was recorded, and if applicable, the species of flora with which they interacted. Additionally, we used six Bushnell camera traps that were active during all the days and nights, and placed at sites previously selected based on their potential mammals attraction (e.g. abundance of food). Each camera was attached to trees at a height of 40cm above the ground and programmed to take 3 photos and a 3-second video per record. Finally, 14 Sherman traps were placed and baited with a mixture of peanut butter, oatmeal and vanilla extract, and similarly located close to the potential sites for the cameras (Fig. 1). Camera traps and Sherman traps were checked during the four sampling days, and baited for the Sherman traps were replaced each day. Furthermore, interactions of mammals with the flora were recorded, including any type of use with it (e.g. consumption of fruits or leaves, use of burrows, resting time, use for transfer of tree species) Subsequently, various statistical analyses were carried out following Valdez et al. (2018) to determine the structure and diversity of mammals in the refuge.
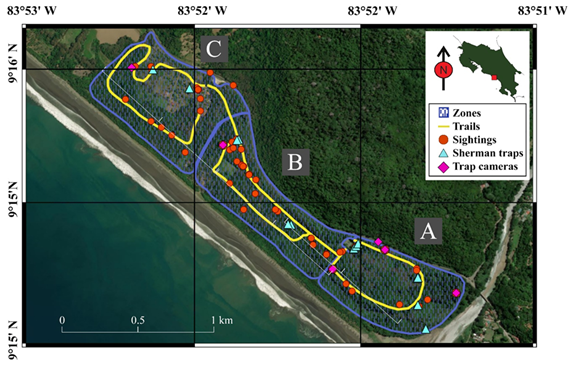
Fig. 1. Hacienda Barú National Wildlife Refuge: zones, trails, and techniques for this study.
In total, 17 different species were sighted, distributed in 13 families and 16 genera. The most frequently sighted species was Cebus imitador with an average of 26 individuals per zone and up to a maximum of 37, followed by Pecari tajacu with a maximum of 53 individuals (Table 1). Also, 17 species of flora were found being used by the mammals (Fig. 2) Number of mammals sightings varied by each technique and depending on each zone (Table 1). Specifically, camera traps were able to record some species not sighted during trail walks (Fig. 3), but also missing other species sighted during trail walks. Sherman traps had a cero rate of captures except for a common crab, baits were found to be intact in all traps.
Interactions of mammals with the observed flora during trails walks were in easy to document since some mammals showed no human avoidance when distance was maintained (Fig. 2). Furthermore, camera traps added more information when species showed human avoidance during trail walks (Fig. 3). Kruskal-Wallis test showed no significant differences between the means of the three zones (H’ = 1,083, p= 0,5467), while the Dominance index reports D= 0,27, 0,30, 0,36 respectively for Zones A, B, and C, being the last one with greater dominance. The richness values for zone A, B, and C, were respectively for the index Margalef DMg= 1,80, 2,10, and 2,20; for Menhinick DMn= 1,10, 9,91, and 1,44; for the alpha diversity with the index of Wiener H’= 1,54, 1,51, and 1,46; and finally 0,72, 0,70, and 0,63 for the Simpson index (1 - D).
Our results demonstrate how effective was the methodology used to undertake a rapid assessment of mammals in Hacienda Barú. Despite that our study was not focused to a specific species, we were able to report more individuals of C. imitador than Gomez-Romero (2020), a studied assessed in the same time period. Here we found that camera traps positively supported data collection for Zone A and B, by adding more species and increasing abundance observations for each zone. Although use of camera traps has been found to get higher detection probability of group-living vs solitary species (Treves et al., 2010; Moore et al., 2020), here camera traps did register more solitary than group-living species. In contrary, null species observations were obtained with the camera trap used for Zone C, but supplemented with the sightings gathered by trail walks.
Table 1
Species detected by zone and technique.
|
Species |
Number of individuals |
|||||
|
Zone A |
Zone B |
Zone C |
||||
|
Camera |
Walking |
Camera |
Walking |
Camera |
Walking |
|
|
Bradypus variegatus |
0 |
1 |
0 |
2 |
0 |
2 |
|
Didelphis marsupialis |
0 |
0 |
0 |
3 |
0 |
2 |
|
Caluromys derbianus |
0 |
0 |
0 |
1 |
0 |
0 |
|
Choloepus hoffmanni |
0 |
2 |
0 |
1 |
0 |
0 |
|
Potos flavus |
0 |
0 |
0 |
1 |
0 |
0 |
|
Nasua narica |
0 |
0 |
17 |
1 |
0 |
2 |
|
Procyon lotor |
4 |
1 |
0 |
0 |
0 |
0 |
|
Cebus imitator |
0 |
21 |
0 |
37 |
0 |
22 |
|
Dasyproctata punctata |
4 |
2 |
4 |
1 |
0 |
7 |
|
Pecari tajacu |
17 |
0 |
0 |
53 |
0 |
1 |
|
Cuniculus paca |
0 |
0 |
2 |
0 |
0 |
1 |
|
Canis latrans |
1 |
0 |
0 |
0 |
0 |
0 |
|
Leopardus pardalis |
1 |
0 |
0 |
0 |
0 |
0 |
|
Sciurius variegatoides |
0 |
0 |
0 |
0 |
0 |
2 |
|
Proechimys semispinosus |
0 |
0 |
0 |
0 |
0 |
1 |
|
Galictis vittata |
0 |
0 |
0 |
0 |
0 |
1 |

Fig. 2. Global representation of mammal species interactions with the surrounding flora.

Fig. 3. Camera traps: Leopardus pardalis (A), Canis latrans (B), Pecari tajacu (C), and Dasyprocta punctata (D).
In the case of the Sherman traps, there could be some considerations related to the cero captures of species of interest. Used bait might not be the adequate to attract mammals, or for instance the chosen sites where the traps were placed were not convenient. Others studied where captures were successful used different bait or placed traps at different heights (e.g. Fialho et al., 2019). The documented relation of some mammals with species of flora within the reserve, also provides a first insight of which species of flora are needed to improve conditions for mammal species. This can be considered if actions of reforestation and conservation are wanted. Despite no significant differences were found between zones, Hacienda Barú should continue limiting tourism access to just Zone C to prevent changes in mammal’s behavior by human activities in Zones A and B.
To the authors consideration, this is the first study of mammals in Hacienda Barú and surroundings. Despite the short time of data collection, we provided a list of mammals species that can occur in Hacienda Barú. This work gives base information for further studies with mammals in the area, and assistance to the Hacienda Barú effort in protecting their territory. We recommend long-term studies to better understand the assemblage of mammals in Hacienda Barú.
ACKNOWLEDGEMENTS
We thank Esmeralda Arevalo-Huezo, Ronald Villalobos Hoffmann, and Gabriela Chavarria-Soley for their support in this research, and to Hacienda Barú National Wildlife Refuge for allowing to carry out this study.
ETHICAL, CONFLICT OF INTEREST AND FINANCIAL STATEMENTS
The authors declare that they have fully complied with all pertinent ethical and legal requirements, both during the study and in the production of the manuscript; that there are no conflicts of interest of any kind; that all financial sources are fully and clearly stated in the acknowledgements section; and that they fully agree with the final edited version of the article. A signed document has been filed in the journal archives.
The statement of each author’s contribution to the manuscript is as follows: D.F-M.: Study design, data collection, analysis, writing and manuscript preparation, and final approval of the manuscript. C.S-G., D.G-M., M.C-A., M.P-L.: Study design, data collection, analysis, and final approval of the manuscript.
REFERENCIAS
Andresen, E., Arroyo-Rodríguez, V., & Ramos-Robles, M. (2018). Primate seed dispersal: old and new challenges. International Journal of Primatology, 39(3), 443-465. https://doi.org/10.1007/s10764-018-0024-z
Bogoni, J. A., Cherem, J. J., Hettwer Giehl, E. L., Oliveira-Santos, L. G., de Castilho, P. V., Picinatto, V., Moreli, F., Tortato, M., Ribeiro, M., & Graipel, M. E. (2016). Landscape features lead to shifts in communities of medium-to large-bodied mammals in subtropical Atlantic Forest. Journal of Mammalogy, 97(3), 713-725. https://doi.org/10.1093/jmammal/gyv215
Ceballos, G., Ehrlich, P. R., & Dirzo, R. (2017). Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proceedings of the national academy of sciences, 114(30), E6089-E6096. https://doi.org/10.1073/pnas.1704949114
Crandall, K. A., Bininda-Emonds, O. R., Mace, G. M., & Wayne, R. K. (2000). Considering evolutionary processes in conservation biology. Trends in ecology & evolution, 15(7), 290-295. https://doi.org/10.1016/S0169-5347(00)01876-0
Dudley, N., Parrish, J. D., Redford, K. H., & Stolton, S. (2010). The revised IUCN protected area management categories: the debate and ways forward. Oryx, 44(4), 485-490. doi:10.1017/S0030605310000566
Gomez-Romero, M. (2020). Dinámica Poblacional y dieta del Cebus capucinus (Primates: Cebidae), en el refugio de vida silvestre Barú, Puntarenas, Costa Rica. Revista Ecología y Desarrollo Sostenible, 2, 1-15. https://revistas.ulatina.ac.cr/index.php/ecologia/article/view/343
Hagger, V., Fisher, D., Schmidt, S., & Blomberg, S. (2013). Assessing the vulnerability of an assemblage of subtropical rainforest vertebrate species to climate change in south‐east Queensland. Austral Ecology, 38(4), 465-475. https://doi.org/10.1111/j.1442-9993.2012.02437.x
Holdridge, L. R. (1987). Ecología basada en zonas de vida (No. 83). Agroamérica.
Fedriani, J. M., & Delibes, M. (2009). Seed dispersal in the Iberian pear, Pyrus bourgaeana: a role for infrequent mutualists. Ecoscience, 16(3), 311-321. https://doi.org/10.2980/16-3-3253
Fialho, M. Y., Cerboncini, R. A., & Passamani, M. (2019). Linear forest patches and the conservation of small mammals in human-altered landscapes. Mammalian Biology, 96(1), 87-92. https://doi.org/10.1016/j.mambio.2018.11.002
Jules, E. S., & Shahani, P. (2003). A broader ecological context to habitat fragmentation: why matrix habitat is more important than we thought. Journal of Vegetation Science, 14(3), 459-464. https://doi.org/10.1111/j.1654-1103.2003.tb02172.x
Ladle, R., Jepson, P., Malhado, A., Jennings, S., & Barua, M. (2011). The causes and biogeographical significance of species’ rediscovery. Frontiers of Biogeography, 3(3), 111-118. https://doi.org/10.21425/F5FBG12432
Laurance, W. F., Sayer, J., & Cassman, K. G. (2014). Agricultural expansion and its impacts on tropical nature. Trends in ecology & evolution, 29(2), 107-116. https://doi.org/10.1016/j.tree.2013.12.001
Li, W., Yang, P., Li, B., Liu, C., Sun, L., & Li, J. (2021). Habitat characteristics or protected area size: What is more important for the composition and diversity of mammals in nonprotected areas?. Ecology and Evolution, 11(12), 7250-7263. https://doi.org/10.1002/ece3.7540
Meyer, C., Kreft, H., Guralnick, R., & Jetz, W. (2015). Global priorities for an effective information basis of biodiversity distributions. Nature communications, 6(1), 1-8. https://doi.org/10.1038/ncomms9221
Moore, J. F., Pine, W. E., Mulindahabi, F., Niyigaba, P., Gatorano, G., Masozera, M. K., & Beaudrot, L. (2020). Comparison of species richness and detection between line transects, ground camera traps, and arboreal camera traps. Animal Conservation, 23(5), 561-572. https://doi.org/10.1111/acv.12569
Newbold, T., Hudson, L. N., Hill, S., Contu, S., Lysenko, I., Senior, R. A., Börger, L., Bennett, D. J., Choimes, A., Collen, B., Day, J., De Palma, A., Díaz, S., Echeverria-Londoño, S., Edgar, M. J., Feldman, A., Garon, M., Harrison, M., Alhusseini, T., Ingram, D. J., Itescu, Y., ... & Purvis, A. (2015). Global effects of land use on local terrestrial biodiversity. Nature, 520(7545), 45-50. https://doi.org/10.1038/nature14324
Passamani, M., & Fernandez, F. A. S. (2011). Abundance and richness of small mammals in fragmented Atlantic Forest of southeastern Brazil. Journal of Natural History, 45(9-10), 553-565. https://doi.org/10.1080/00222933.2010.534561
Smith, T. B., Kark, S., Schneider, C. J., Wayne, R. K., & Moritz, C. (2001). Biodiversity hotspots and beyond: the need for preserving environmental transitions. Trends in Ecology & Evolution, 16(8), 431.
Stolton, S., Redford, K. H., & Dudley, N. (2014). The futures of privately protected areas. IUCN.
The Weather Channel. (2023). Tiempo Mensual. An IBM Business. https://weather.com/
Treves, A., Mwima, P., Plumptre, A. J., & Isoke, S. (2010). Camera-trapping forest-woodland wildlife of western Uganda reveals how gregariousness biases estimates of relative abundance and distribution. Biological Conservation, 143(2), 521-528. https://doi.org/10.1016/j.biocon.2009.11.025
UNEP-WCMC & IUCN. (2016). Protected Planet Report 2016. https://bit.ly/3ppK8MP
Valdez, C. G., Guzmán, M. A., Valdés, A., Forougbakhch, R., Alvarado, M. A., & Rocha, A. (2018). Estructura y diversidad de la vegetación en un matorral espinoso prístino de Tamaulipas, México. Revista de Biología Tropical, 66(4), 1674-1682. http://dx.doi.org/10.15517/rbt.v66i4.32135
Volenec, Z. M., & Dobson, A. P. (2020). Conservation value of small reserves. Conservation Biology, 34(1), 66-79. https://doi.org/10.1111/cobi.13308
