
In vitro life table of the storage mite Tyrophagus putrescentiae (Acari: Acaridae)
Pamela
Murillo1![]() & Hugo Aguilar1
& Hugo Aguilar1![]()
1. Universidad de Costa Rica, Escuela de Agronomía, Centro de Investigación en Protección de Cultivos, Laboratorio de Acarología. P.O. Box 2060, San Pedro de Montes de Oca, San José, Costa Rica; pamela.murillorojas@ucr.ac.cr, hugo.aguilar@ucr.ac.cr
Received 20-VII-2022 Corrected 10-XI-2022 Accepted 4-I-2023
DOI: https://doi.org/10.22458/urj.v15i1.4335
|
ABSTRACT. Introduction: The mite Tyrophagus putrescentiae is a common contaminant of stored products and an important pest in plant tissue culture laboratories, because they diseminate fungi and bacteria. Objective: To describe the reproduction and life table of T. putrescentiae in vitro. Methods: We reared the mites with its associated fungus Leptosphaerulina sp., recording growth and development every 12h. Results: The duration of the egg, larva, protonymph, and tritonymph stages of cohort 1 was 4,52; 1,57; 1,37 and 1,29 days (cohort 2: 4,54; 1,44; 1,31 and 1,45 days, respectively). Cohort 1 periods of pre-oviposition, oviposition and post-oviposition were 1,86; 7,21 and 1,35 days (most cohort 2 individuals did not reach maturity). The intrinsic rate of natural increase (rm) was 0,11 individuals per female per day, the net reproduction rate (Ro) was 29,21; the generation time was 29,47 days, and the finite rate of increase (λ) was 1,12 times per female per day. Conclusion: Under the typical laboratory conditions, T. putrescentiae can multiply its initial population in a single day, which explains the population explosions observed in these laboratories.
Keywords: Biological parameters, life table, in vitro plants, laboratory conditions, culture pest control.
|
RESUMEN. “Tabla de vida in vitro del ácaro Tyrophagus putrescentiae (Acari: Acaridae) en condiciones de almacenamiento”. Introducción: El ácaro Tyrophagus putrescentiae es un contaminante común de los productos almacenados y una plaga importante en los laboratorios de cultivo de tejidos vegetales, ya que disemina hongos y bacterias. Objetivo: Describir la reproducción y tabla de vida de T. putrescentiae in vitro. Métodos: Criamos los ácaros con su hongo asociado Leptosphaerulina sp., registrando el crecimiento y desarrollo cada 12h. Resultados: La duración de los estadios de huevo, larva, protoninfa y tritoninfa de la cohorte 1 fue de 4,52; 1,57; 1,37 y 1,29 días (cohorte 2: 4,54; 1,44; 1,31 y 1,45 días, respectivamente). Los períodos de preoviposición, oviposición y postoviposición de la cohorte 1 fueron 1,86; 7,21 y 1,35 días (la mayoría de los individuos de la cohorte 2 no alcanzaron la madurez). La tasa intrínseca de crecimiento natural (rm) fue de 0,11 individuos por hembra por día, la tasa neta de reproducción (Ro) fue de 29,21; el tiempo de generación fue de 29,47 días, y la tasa finita de incremento (λ) fue de 1,12 veces por hembra por día. Conclusión: En las condiciones típicas de laboratorio, T. putrescentiae puede multiplicar su población inicial en un solo día, lo que explica las explosiones de población observadas en estos laboratorios.
Palabras clave: Parámetros biológicos, tabla de vida, plantas in vitro, condiciones de laboratorio, control de plagas de cultivo.
|
Plant tissue culture is done under aseptic conditions and in general, involves the massive multiplication of plants in a culture medium (Leifert et al., 1991; Conger, 2018). For some plant species, this method has advantages over other production systems, e.g. growing varieties in large quantities in a short period of time (Villalobos & Thorpe, 1991).
A requirement to succeed in the micro-propagation of any plant species is to keep the glass containers (vessels) free of contaminants like fungi and bacteria. In many cases, these pathogens are introduced by small arthropods such as mites, which can carry and disseminate microbial contaminants on their bodies, causing the total or partial loss of the vegetative material (Cassells, 2000; Odutayo et al., 2007; Murillo-Rojas & Aguilar-Piedra, 2021).
The mold mite, Tyrophagus putrescentiae (Schrank, 1781), is a ubiquitous mite mostly known for infesting stored products, as well as being an important component of the Acari-fauna of house dust (Hughes, 1976; Emmanouel et al., 1994; Fan & Zhang, 2007a; 2007b; OConnor, 2009). It has been identified as one of the major pests of tissue culture laboratories, increasing their populations rapidly and spreading fungi and bacteria within the glass containers (Duek et al., 2001; van Epenhuijsen & Koolaard, 2004; Murillo-Rojas & Aguilar-Piedra, 2021; Murillo et al., 2021).
The biology of T. putrescentiae has been studied under different conditions; however, results of the life history of the mite showed important differences depending on the environmental conditions, gut microbiota, and diet (Ždárková & Voráček, 1993; Duek et al., 2001; Smrž, 2003; Kheradmand et al., 2007; Sánchez-Ramos et al., 2007; Canfield & Wrenn, 2010; Erban et al., 2015; Erban et al. 2016; Rybanska et al., 2016; da Silva et al. 2018). In previous investigations, researchers worked with this species under storage conditions of products such as edible mushrooms, ham, cheese, dry yeast, mouse or dog food, and grains (Sánchez-Ramos & Castañera, 2005; Aygun et al., 2007; Kheradmand et al., 2007; da Silva et al., 2018). However, those scenarios are quite different from what is found in tissue culture laboratories, were the humidity inside the containers is almost 100% and the ingested food by the mites are usually environmental fungi; consequently, the effect of these mites under in vitro conditions are unknown, and might depend on the type of diet supplied, humidity, temperature, and so on.
The level of damage caused by the mites is related to its population size, which depends upon their intrinsic rate of increase or reproductive rate (Murillo et al., 2021). The biological parameters depend upon the survival of the individuals in the population, the rate of development of the immature stages, and the reproductive potential of the adults (Birch, 1948; Bellows et al., 1992; Sánchez-Ramos & Castañera, 2005). Life tables summarize survival and mortality of a population in specific conditions according to the age. Data of fecundity and survival are used to predict changes on the size of a determined population (Tanigoshi & McMurtry, 1977). This research reveals the biological population parameters of T. putrescentiae, which is considered the major contaminant in tissue culture laboratories in Costa Rica.
The main objectives of this investigation were to determine the biology of T. putrescentiae, as well as the life table parameters, simulating in vitro conditions in the plant laboratories where mites might occur to seek, in the future, some practices to manage this contaminant.
MATERIALS AND METHODS
This investigation was carried out in the Laboratory of Acarology, Department of Agronomy, University of Costa Rica. It was developed on two different cohorts, under in vitro conditions.
Live specimens of T. putrescentiae were collected from commercial tissue culture laboratories containers and reared in petri dishes with acidified Potato Dextrose Agar (PDA). Mycelium of the fungus Leptosphaerulina sp. McAlpine (Dothideomycetes: Pleosporaceae), was placed in each plate and maintained at room temperature (22-26oC) for four days allowing the mycelium to cover the PDA medium. This fungus was chosen, as it is commonly found in association with T. putrescentiae and was isolated from most of the containers. Later, mites found in the containers were transfer to the petri dishes and kept here for a month to get a high population.
Biological studies: To determine the life cycle, 20 adult females were chosen from the mite colony, and placed in a petri dish (100 x 15mm) with the fungus mycelium.
Eggs laid by the females during the first 24 hours were separated and transferred to micro plates (Evergreen®) composed by 24 cells per plate. Each cell had 2ml of PDA and the fungus previously established. Forty-eight replicates were established for the first cohort and 30 for the second, according to the methodology proposed by Kheradmand et al. (2007).
Micro plates were sealed with Parafilm® to avoid the entrance or exit of mites. Each plate, with a single egg per cell, was kept at room temperature (20-25°C). The development of all stages was monitored inside a laminar flux chamber to avoid the access of other fungi contaminants on the plates. Evaluations on the life cycle were made every 12 hours with a stereoscope-microscope Optima model ZM-160A.
Data on the incubation period and developmental time of each stage (egg, larva, protonymph, tritonymph and adult) were collected, until the mites reached maturity and died. To calculate sex ratio, females and males were counted when reached maturity.
The ovipositional period was determined by the time elapsed from the emergence of the adult female until the first egg oviposited. Also, we recorded the number of eggs laid per female daily during her life span, whereas the post-oviposition period was measured from the time the female laid her last egg until she died.
Temperature and humidity were recorded every hour with a data logger (HOBO U 12®).
Life table: Once the females reached the adult stage, each one was placed in a petri dish (55 x 15mm) with 10ml of PDA and the fungus. A male was introduced in each plate for 24 hours to allow mating, after which, it was removed. The number of eggs oviposited by each female were counted every 24 hours, until the females died.
To calculate the life table parameters, the program Life-48 (Abou-Setta et al., 1986) was used, which followed the method proposed by Birch (Birch, 1948). From the data obtained in the biological studies above, the population parameters were calculated to construct the life table, i.e., net reproduction rate (Ro= Σmx.lx; mx: total eggs/number of females; lx: live specimens/total specimens), generation time (T= Σmx.lx.x/ Σmx.lx), intrinsic rate of natural increase (rm= log Ro/T.0.4343) and the finite rate of increase (λ= antilog rm). Data were analyzed by the package Statistica 6 (StatSoft, 2001), with the analysis Survival.
RESULTS
Biology: Data showed differences in the developmental time for each mite stages: larva,
protonymph, tritonymph and adult. However, no distinctions were found in all immature stages
between the two cohorts (Table 1).
TABLE 1
Mean development time (days ±SE) of each immature stage of Tyrophagus putrescentiae (Acari: Acaridae), under laboratory conditions.
|
Stage |
Cohort 1 |
Cohort 2 |
|
Egg |
4,52 ± 0,27 |
4,54 ± 0,43 |
|
Larva |
1,57 ± 0,62 |
1,44 ± 0,38 |
|
First molting |
0,47 ± 0,25 |
0,43 ± 0,25 |
|
Protonymph |
1,37 ± 0,51 |
1,31 ± 0,50 |
|
Second molting |
0,43 ± 0,25 |
0,45 ± 0,25 |
|
Tritonymph |
1,29 ± 0,54 |
1,45 ± 0,67 |
|
Third molting |
0,47 ± 0,28 |
0,51 ± 0,23 |
|
Total |
10,12 ± 1,44 |
10,13 ± 1,44 |
Egg: The average time was 4,52 days for the first cohort and 4,54 days for the second, being the eggs the immature stage with the longest life time cycle (Table 1).
Larva: For the first cohort, the larval stage lasted an average of 1,57 days, whereas the second cohort last an average of 1,44 days (Table 1).
Protonymph: The average time was 1,37 and 1,31 days for the first and second cohorts, respectively (Table 1).
Tritonymph: The average duration for the first and second cohorts were 1,29 and 1,45 days, respectively (Table 1).
Eggs showed to have the highest survival of all other immature stages. In general, when immature molt to a different stage their survival rate decreases (Fig. 1A).
Adult: Sixty eight percent of mites from cohort 1 reached the adult stage (egg-adult) with an average generational time of 10,41 days (Table 2). However, only 20% of the mites from cohort 2 (the smaller cohort) reach maturity; therefore, the recording data on this population did not continue.
The survival rate of females diminished throughout time. Consequently, on day 10, about 50% of females were still alive, whereas on day 17, only 18% survived (Fig. 1B). The average for the pre-ovipositional period was 1,86 days, the oviposition period 7,21 days and post-oviposition period 1,35 days (Table 2).
Each female deposited on average 3,8 ± 1,64 eggs per day. The higher number laid by all females occurred during the second day with a total of 124 eggs (Fig. 1C). Most eggs were laid during the first eight days, however after this date, a decrease in their oviposition rate was observed, having a steady decline in the last five days (Fig. 1C).
Molting: The duration of each of the three molting stages was similar in the first and second cohorts. For cohort 1, the first molts averaged 0,47, the second 0,43, and the third 0,47 days, whereas that of cohort 2 averaged 0,43; 0,45 and 0,51 days, respectively.
TABLE 2
Duration time (days ±SE) of pre-oviposition, oviposition, post-oviposition for females of Tyrophagus putrescentiae (Acari: Acaridae) under laboratory conditions for Cohort 1.
|
Period |
Number of days |
|
Pre-oviposition |
1,86 ± 1,04 |
|
Oviposition |
7,21 ± 5,02 |
|
Post-oviposition |
1,35 ± 1,52 |
|
Total longevity of females |
10,41 ± 3,93 |
Life table: The population parameters for T. putrescentiae are the intrinsic rate of natural increase was 0,11 individuals per female per day, and the net reproduction rate 29,21. Moreover, T. putrescentiae showed a generational time of 29,47 and a finite rate of increase of 1,12.
Considering these numbers, T. putrescentiae can increase itself 29,21 times in a generation time of 29,47 days, under the environmental conditions of this investigation, with an intrinsic rate of natural increase of 0,11 individuals per female per day. The finite rate of increase was 1,12, meaning that the population multiplies itself 1,12 times in one day.
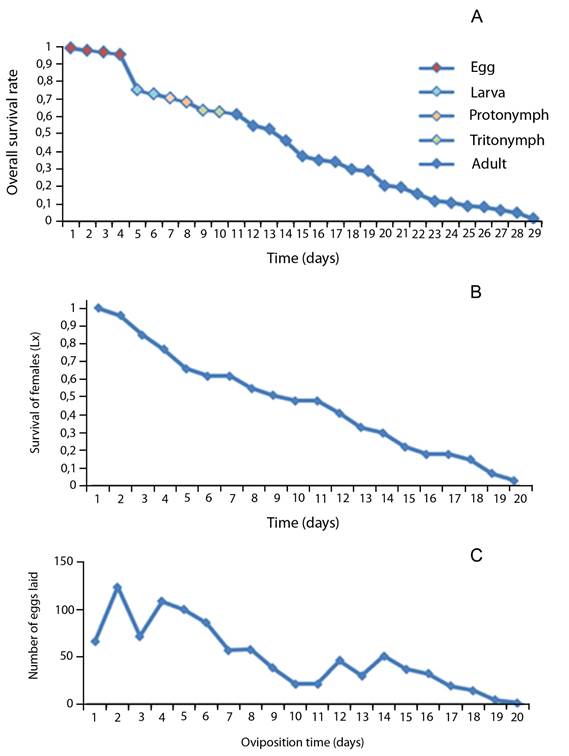
Fig. 1. Life cycle survival rate (A), adult female survival rate (B), and number of eggs oviposited by females (C) of Tyrophagus putrescentiae under in vitro conditions.
DISCUSSION
In this study, the developmental periods for T. putrescentiae (Table 1) were shorter compared to other acarid mites reared under distinct conditions, such as stored products, probably the in vitro conditions may have led to a reduction in the life cycle of the mite (Rojas, 1992; Kasuga & Amano, 2000; Kheradman et al., 2007; Xia et al., 2009). In our research, the larval stage lasted an average of 1,57 days for cohort 1 and 1,44 for cohort 2 (Table1), whereas other investigators have reported values ranging from 2,1 to 2,5 days (Kasuga & Amano, 2000; Sánchez-Ramos & Castañera, 2005; Kheradman et al., 2007; Sánchez-Ramos et al., 2007). This reduction in the developmental time is probably one of the reasons why these laboratories confront steady and exponential population growth under in vitro conditions.
However, our study also found some similarities with other results related to cohort Astigmatina. For example, Xia et al. (2009) found that although the duration of the diverse stages of Aleuroglyphus ovatus Troupeau (Acari: Acaridae), varies according to the temperature, the egg was always the immature stage with the lengthiest interval at all temperatures evaluated. Similarly, in our case, the egg was the lengthy immature stage, lasting much longer than any other immature stage (Table 1). This could represent a problem under in vitro conditions, given that eggs are less perceptible for workers monitoring these mites and because of the known tolerance of eggs to low temperatures (Eaton & Kells, 2011). Thus, the prevalence of eggs represents a primary source of entrance for mites when plant material arrives from other laboratories.
Similarly, the protonymphal and tritonymphal stages have comparable values to other investigations (Kasuga & Amano, 2000; Sánchez-Ramos & Castañera, 2005; Kheradman et al., 2007; Sánchez-Ramos et al., 2007). As it can be observed on Table 1, these differences in the development of immature stages of Tyrophagus, showed a notable reduction in the life cycle under the conditions established for this investigation. Several authors mention that the developmental time of each immature stage of T. putrescentiae can be affected by factors such as temperature, relative humidity, and type of food consumed (Abdel-Sater et al., 1995; Sánchez-Ramos & Castañera, 2005; Kheradman et al., 2007; Sánchez-Ramos et al., 2007; Erban et al., 2016; Rybanska et al., 2016; da Silva et al., 2018). Kheradman et al., 2007 noticed that the developmental period for this mite, at the same temperature (25oC), was affected by the fungal species used to raise the colonies; they observed a longer life span when mites were fed on oyster fungus Pleurotus ostreatus (Jacq.) P. Kumm. than other fungus. Consequently, rearing the mites on the fungus Leptosphaerulina sp., could have had a significant effect shortening the time of the immature stages of Tyrophagus, enabling more generations within a reduced period.
The average duration for the developmental periods of the adult females of T. putrescentiae, pre-oviposition, oviposition, and post-oviposition (Table 2), also varies as much as those for immature stages when compared to other acarid mites (Gerson et al., 1984; Rojas, 1992; Sánchez-Ramos & Castañera, 2005; Bahrami et al., 2007; Kheradman et al., 2007; Sánchez-Ramos et al., 2007; Xia et al., 2009). Factors as temperature, relative humidity, and food type also influence the longevity and fecundity of the females (Kheradmand et al., 2007). Like this, Gerson et al., (1984) observed that food type had a direct effect on the oviposition of the acarid mite Rizoglyphus robini Claparède (Acari:Acaridae) and found that females reared on a diet based on peanuts began the oviposition period one day after mating, while those fed on garlic, began their period two days after copulation.
The fecundity data obtained for T. putrescentiae under this investigation, are in accordance with the typical pattern described for other acarid mites, such as Acarus farris Oudemans (Sánchez-Ramos & Castañera, 2007), Aleuroglyphus ovatus (Xia et al., 2009), T. similis Volgin (Kasuga & Amano, 2000) and T. putrescentiae (Sánchez-Ramos & Castañera ,2005; Bahrami et al., 2007). Females laid a maximum number of eggs during the first days of oviposition and showed a high rate of fecundity sustained for a short period of time, decreasing rapidly at the end (Fig. 1C). Although the females oviposited their eggs during a short period of time, the large number of eggs laid by each female is perhaps the key factor that allows these mites to increase their population so quickly.
Considering the susceptibility of these mites to low relative humidity and extreme temperatures, management practices to control these arthropods on storage products, have obtained good results when one of these two factors are changed. However, when dealing with plant tissue culture, is not easy to adjust those parameters as plants also depend on temperature and humidity to survive. Thus, the manipulation of temperature and relative humidity for the control of Tyrophagus mites within an in vitro system, is not a viable option, nowadays.
According to the results obtained on the life table for this species, it was determined that T. putrescentiae may be able to increase its population 29,21 times in a generational time of 29,47 days, with an increasing rate of natural increase of 0,11 individuals per female per day. The finite rate of increase was 1,12, suggesting that the population can multiply itself 1,12 times in only one day, under the conditions established for this investigation. Even though this mite shows a great capacity to increase its population in a short period of time, several authors have found both higher and lower values for the different population parameters, indicating the high adaptative capacity to different conditions in which they grow (Bahrami et al., 2007; Kheradmand et al., 2007; da Silva et al., 2018).
Considering other examples of acarid mites such as Rhizoglyphus robini, and Tyrophagus species, the values for the population parameters may greatly vary when environmental factors changed. In general, populations did not disappear completely but, are adapted to the diverse conditions on which they develop, indicating the high survival capacity of acarids. For example, Gerson et al., (1984) noticed differences on the intrinsic rate of natural increase of R. robini at 27oC when the diet shifted. Mites fed on an artificial diet based on peanuts had an increasing rm rate of 0,285, whereas when fed on garlic, they observed a reduction of the rm to 0,218. Nevertheless, this variation of food did not eliminate the population.
Our study suggest that Tyrophagus is able to adjust its population parameters according to the temperature and diet. Thus, it is recommended to perform further studies on this species under in vitro conditions but offering other diets, as it is known that different fungi species grow inside the culture medium used for in vitro culture, which can serve as food for T. putrescentiae (Parkinson et al., 1991; Abdel-Sater et al., 1995).
Comparing the results obtained in this investigation with the population parameters of other experiments, we conclude that diet, as well as temperature, have a strong effect on the population of T. putrescentiae. Based on the values of life table for T. putrescentiae under in vitro conditions, it was found that these mites have a high population replacement rate, which allows them to reach their high potential as a pest under these conditions. In addition, it is necessary to consider the initial population size of infestation in the tissue culture laboratories, as the population can reach high numbers inside the containers, generating significant economic losses. Similarly, it is important to consider that plants growing in vitro are isolated in the vessels for around 3 months, which allows mites to become established and rapidly increase their population.
ACKNOWLEDGEMENTS
We thank all the vitro culture laboratories that help us with the material for this study.
ETHICAL, CONFLICT OF INTEREST AND FINANCIAL STATEMENTS
The authors declare that they have fully complied with all pertinent ethical and legal requirements, both during the study and in the production of the manuscript; that there are no conflicts of interest of any kind; that all financial sources are fully and clearly stated in the acknowledgements section; and that they fully agree with the final edited version of the article. A signed document has been filed in the journal archives.
The statement of each author’s contribution to the manuscript is as follows: P.M.R. and H.A.P: Study design, analysis, preparation and final approval of the manuscript. PMR: Data collection.
REFERENCES
Abdel-Sater, M. A., Hemida, S. K., & Eraky, S. A. (1995). Distribution of fungi on two mite species and their habitats in Egypt. Folia Microbiologica, 40, 304-313. https://doi.org/10.1007/BF02814214
Abou-Setta, M. M., Sorrell, R. W., & Childers, C. C. (1986). Life 48: A basic computer program to calculate life table parameters for an insect or mite species. Florida Entomologist, 69(4), 690-697. https://doi.org/10.2307/3495215
Aygun, O., Yaman, M., & Durmaz, H. (2007). A survey on occurrence of Tyrophagus putrescentiae (Acari: Acaridae) in Surk, a traditional Turkish dairy product. Journal of Food Engineering, 78(3), 878-881. https://doi.org/10.1016/j.jfoodeng.2005.11.029
Bahrami, F., Kamali, K., & Fathipour Y. (2007). Life history and population growth parameters of Tyrophagus putrescentiae (Acari: Acaridae) on Fusarium graminearum in laboratory conditions. Journal of Entomological Society of Iran, 26(2), 7-18.
Bellows, T. S., Van Driesche, R. G., & Elkinton, J. S. (1992). Life table construction and analysis in the evaluation of natural enemies. Annual Review of Entomology, 37, 587-614. https://doi.org/10.1146/annurev.en.37.010192.003103
Birch, L.C. (1948). The intrinsic rate of natural increase of an insect population. Journal of Animal Ecology, 17(1), 15-26. https://doi.org/10.2307/1605
Canfield, M. S., & Wrenn, W. J. (2010). Tyrophagus putrescentiae mites grown in dog food cultures and the effect mould growth has on mite survival and reproduction. Veterinary Dermatology, 21(1), 58-63. https://doi.org/10.1111/j.1365-3164.2009.00778.x
Cassells, A. C. (2000). Aseptic microhydroponics: a strategy to advance microplant development and improve microplant physiology. Acta Horticulturae, 530, 187-194. https://doi.org/10.17660/ActaHortic.2000.530.21
Conger, B. V. (2018). Cloning agricultural plants via in vitro techniques. CRC Press Taylor & Francis Group.
da Silva, G.L., Esswein, I. Z., de Souza Radaelli, T. F., Rocha, M. S., Ferla, N., & da Silva, O. S. (2018). Influence of various diets on development, life table parameters and choice oviposition test of Tyrophagus putrescentiae (Acari: Acaridae): An illustration using scanning electron microscopy (SEM). Journal of Stored Products Research, 76, 77-84. https://doi.org/10.1016/j.jspr.2018.01.006
Duek, L., Kaufman, G., Palevsky E., & Berdicevsky I. (2001). Mites in fungal cultures. Mycoses, 44(9-10), 390-394. https://doi.org/10.1046/j.1439-0507.2001.00684.x
Eaton, M., & Kells, S. A. (2011). Freeze mortality characteristics of the mould mite Tyrophagus putrescentiae, a significant pest of stored products. Journal of Economic Entomology, 104(4), 1423-1429. https://doi.org/10.1603/EC10429
Emmanouel, N. G., Buchelos, C. T., & Dukidis, E. (1994). A survey on the mites of stored grain in Greece. Journal of Stored Product Research, 30(2), 175-178. https://doi.org/10.1016/0022-474X(94)90196-1
Erban, T., Rybanska, D., & Hubert, J. (2015). Population growth of the generalist mite Tyrophagus putrescentiae (Acari: Acaridida) following adaptation to high- or low-fat and high- or low-protein diets and the effect of dietary switch. Environmental Entomology, 44(6), 1599-1604. https://doi.org/10.1093/ee/nvv129
Erban, T., Klimov, P.B., Smrz, J., Phillips, T. W., Nesvorna, M., Kopecky, J., & Hubert, J. (2016). Populations of stored product mite Tyrophagus putrescentiae differ in their bacterial communities. Frontiers Microbiology, 7, 1046. https://doi.org/10.3389/fmicb.2016.01046
Fan, Q-H. & Zhang, Z-Q. (2007a). Tyrophagus (Acari: Astigmata: Acaridae) Fauna of New Zealand. Manaaki Whenua Press. https://doi.org/10.7931/J2/FNZ.56
Fan, Q-H. & Zhang, Z-Q. (2007b). Revision of some species of Tyrophagus (Acari: Acaridae) in Oudemans Collection. Systematic and Applied Acarology, 12, 253-280. https://doi.org/10.11158/saa.12.3.11
Gerson, H., Capua, S., & Thorens D. (1984). Life history and life tables of Rhizoglyphus robini Claparède (Acari: Astigmata: Acaridae). Acarologia, 24(4), 439-448. https://bit.ly/3WTr8Be
Hughes, A. M. (1976). The mites of Stored Food and Houses (2nd ed.). Her Majesty´s Stationary Office.
Kasuga, S., & Amano, H. (2000). Influence of temperature on the life history parameters of Tyrophagus similis Volgin (Acari: Acaridae). Applied Entomology Zoology, 35(2), 237-244. https://doi.org/10.1303/aez.2000.237
Kheradmand, K., Kamali, K., Fathipour, Y., & Mohammadi-Goltapeh E. (2007). Development, life table and thermal requirement of Tyrophagus putrescentiae (Astigmata: Acaridae) on mushrooms. Journal of Stored Products Research, 43, 276-281. https://doi.org/10.1016/j.jspr.2006.06.007
Leifert, C., Ritchie, J., & Waites, W. M. (1991). Contaminants of plant tissue and cell cultures. World Journal of Microbiology and Biotechnology, 7, 452-469. https://bit.ly/3wOnUUT
Murillo-Rojas, P., & Aguilar-Piedra, H. (2021). Principales ácaros encontrados en laboratorios comerciales de cultivo de tejidos vegetales y su asociación con hongos en el Valle Central de Costa Rica. Agronomía Costarricense, 45(1), 41-52. https://doi.org/10.15517/RAC.V45I1.45679
Murillo, P., Arias, J., & Aguilar, H. (2021). First record and verification of Tyrophagus putrescentiae (Acari: Acaridae) causing direct damage on anthurium plants cultivated in vitro. Systematic and Applied Acarology, 26(11), 2048–2058. https://doi.org/10.11158/saa.26.11.5
OConnor, B. M. (2009). Cohort Astigmatina.. In G.W. Krantz & D.E. Walter (Eds). A manual of Acarology (3rd Ed., pp.565-657). Texas Tech University press.
Odutayo, O. I., Amusa, N. A., Okutade O. O., & Ogunsanwo Y. R. (2007). Sources of microbial contamination in tissue culture laboratories in southwestern Nigeria. African Journal of Agricultural Research, 2(3), 67-72.
Parkinson, C. L., Jamieson, N., Eborall, J., & Armitage, D. M. (1991). Comparison of the fecundity of three species of grain store mites on fungal diets. Experimental and Applied Acarology, 12, 297-302. https://doi.org/10.1007/BF01193474
Rojas, E. (1992). Bionomics of three species of fungivorous mites (Acari), Tarsonemus sp. (Prostigmata: Tarsonemidae), Tyrophagus putrescentiae (Schrank), Caloglyphus sp. (Astigmata: Acaridae) and their natural enemy, Lasioceius sp. (Mesostigmata: Ascidae). [Master Thesis, Texas A & M University, USA].
Rybanska, D., Hubert, J., Markovic, M., & Erban T. (2016). Dry dog food integrity and mite strain influence the density-dependent growth of the stored-product mite Tyrophagus putrescentiae (Acari: Acaridida). Journal of Economic Entomology, 109(1),454–460. https://doi.org/10.1093/jee/tov298
Sánchez-Ramos, I., & Castañera P. (2005). Effect of temperature on reproductive parameters and longevity of Tyrophagus putrescentiae (Acari: Acaridae) Experimental and Applied Acarology, 36, 93-105. https://doi.org/10.1007/s10493-005-0506-5
Sánchez-Ramos, I., & Castañera, P. (2007). Effect of temperature on reproductive parameters and longevity of Acarus farris (Acari: Acaridae). Journal of Stored Products Research, 43(4), 578-586. https://doi.org/10.1016/j.jspr.2007.03.008
Sánchez-Ramos, I., Álvarez-Alfageme, F., & Castañera, P. (2007). Effects of relative humidity on development, fecundity and survival of three storage mites. Experimental and Applied Acarology, 41, 87-100. https://doi.org/10.1007/s10493-007-9052-7
Smrž, J. (2003). Microanatomical and biological aspects of bacterial associations in Tyrophagus putrescentiae (Acari: Acaridida). Experimental and Applied Acarology, 31, 105-113. https://doi.org/10.1023/b:appa.0000005111.05959.d6
StatSoft Inc. (2001). Statistica for Windows. Version 6. USA.
Tanigoshi, L. K., & McMurtry, J. A. (1977). The dynamics of predation of Stethorus picipes (Coleoptera: Coccinellidae) and Typhlodromus floridanus on the prey Oligonychus punicae (Acarina: Phytoseiidae, Tetranychidae). Part I. Comparative life history and life table studies. Hilgardia, 45(8), 237-261. https://doi.org/10.3733/hilg.v45n08p237
van Epenhuijsen, C. W., & Koolaard J. (2004). Effective aerosol treatment of mould mites and onion thrips in tissue culture. New Zealand Plant Protection, 57, 202-208. https://doi.org/10.30843/nzpp.2004.57.6911
Villalobos, V. M., & Thorpe T. A. (1991). Micropropagación: concepto, metodología y resultados. In W. M. Roca & L. A. Mroginski (Eds), Cultivo de Tejidos en la Agricultura: fundamentos y aplicaciones (pp.127-142). CIA Tropical.
Xia, B., Dongmei, L., Zhiwen, Z., & Zhimin Z. (2009). Effect of temperature on the life cycle of Aleuroglyphus ovatus (Acari: Acaridae) at four constant temperatures. Journal of Stored Products Research, 45, 190-194. https://doi.org/10.1016/j.jspr.2009.02.001
Ždárková, E., & Voráček V. (1993). The effect of physical factors on survival of stored food mites. Experimental and Applied Acarology, 17, 197–204. https://doi.org/10.1007/BF00118436