Biochemical and physiological responses of the tree Pterogine nitens (Fabaceae) to simulated glyphosate drift
Diego
Ariel Meloni1![]() , María José Nieva2
, María José Nieva2![]() , & Ignacio Aspiazú3
, & Ignacio Aspiazú3![]()
1. Universidad Nacional de Santiago del Estero, Facultad de Agronomía y Agroindustrias, INDEAS, Av. Belgrano (S) 1912, Santiago del Estero, Argentina; dmeloniunse@gmail.com
2. Universidad Nacional de Santiago del Estero, Facultad de Ciencias Forestales, INSIMA, Av. Belgrano (S) 1912, Santiago del Estero, Argentina; marijo_nieva@hotmail.com
3. Universidade Estadual de Montes Claros, Janaúba, Minas Gerais, Brazil; Ignacio.aspiazu@unimontes.br
Received 05-IX-2021 Corrected 7-XII-2021 Accepted 13-XII-2021
DOI: https://doi.org/10.22458/urj.v14i1.3825
|
ABSTRACT. Introduction: In the last decades, the natural dispersal area of P. nitens has been subjected to a change in land use. The agricultural frontier has expanded, at the expense of native forest, with the incorporation of productive systems that use high doses of the herbicide glyphosate. The degree of tolerance of the species to the herbicide and its physiological responses are unknown. Objective: To evaluate the biochemical and physiological responses of Pterogine nitens to simulated glyphosate drift. Methods: Greenhouse trials were conducted with one-year-old plants grown in pots. Glyphosate drift was simulated at doses of 0,65 and 130g a.e. ha-1. We measured concentrations of shikimate, photosynthetic pigments, and mineral composition, 20 days after application. Results: Glyphosate increased concentrations of shikimate and decreased concentrations of photosynthetic pigments. It also reduced concentrations of potassium, magnesium, calcium, zinc, manganese, and iron. Conclusions: Glyphosate alters the photochemical stage of photosynthesis by decreasing the concentration of photosynthetic pigments. It also interferes with macro- and micronutrient homeostasis.
Keywords: agrochemicals, ecophysiology, environmental impact, mineral nutrition, abiotic stress
|
RESUMEN. “Respuestas bioquímicas y fisiológicas del árbol Pterogine nitens (Fabaceae) a la deriva simulada de glifosato”. Introducción: En las últimas décadas, el área de dispersión natural de P. nitens, estuvo sometida a un cambio en el uso de la tierra. Se expandió la frontera agropecuaria, a expensas del bosque nativo, con la incorporación de sistemas productivos que utilizan altas dosis del herbicida glifosato. Se desconoce el el grado de tolerancia de la especie al herbicida, y su respuestas fisiológicas. Objetivo: Evaluar las respuestas bioquímicas y fisiológicas de Pterogine nitens a la deriva simulada de glifosato. Métodos: Hicimos ensayos en invernáculo, con plantas de un año de edad, en macetas. Simulamos la deriva de glifosato, en dosis de 0,65 y 130g e.a. ha-1. Veinte días después de la aplicación, medimos las concentraciones de shikimato, pigmentos fotosíntéticos, y la composición mineral. Resultados: El glifosato incrementó las concentraciones de shikimato, y disminuyó las concentración de pigmentos fotosintéticos. También redujo las cocentraciones de potasio, magnesio, calcio, zinc, manganeso y hierro. Conclusiones: El glifosato altera la etapa fotoquímica de la fotosíntesis, al diminuir la concentración de pigmentos fotosintéticos. También interfiere con la homeostasis de macro y micronutrientes.
Palabras clave: agroquímicos, ecofisiología, impacto ambiental, nutrición mineral, estrés abiótico
|
Tipa colorada (Pterogyne nitens) is a species of forest importance that belongs to the Fabaceae family, subfamily Cesalpinoideae. It is distributed from southern Brazil to Argentina, in the phytogeographic regions of the Yungas, Eastern Chaco District, and Paranaense province (Calzón & Giménez, 2011). According to the International Union for Conservation of Nature, it is in a "near threatened" conservation status (Espíndola et al., 2018). It has fine-textured wood, with yellowish brown sapwood, pinkish brown to reddish brown heartwood, and very attractive veined grain, similar to mahogany. It is used for furniture, fine carpentry, lamination, and tongue and groove joinery (Giménez & Moglia, 2003). It is a tree with semipersistent foliage, long and sinuous stem (7 to 15 m), heliophilous, which has a pioneering role in degraded sites (Giménez & Moglia, 2003).
In recent decades, the natural dispersal area of P. nitens has been subjected to a change in land use. The agricultural frontier expanded at the expense of native forest, with the incorporation of production systems that use high doses of the herbicide glyphosate. As a consequence of this process, soil contamination and loss of biodiversity have been reported, mainly in the Yungas and Gran Chaco regions (Choumert & Phélinas, 2015). On the other hand, the herbicide applied on crops can reach nontarget species of the surrounding vegetation, a phenomenon known as drift (Gomes et al., 2014a).
Glyphosate, N-(phosphonomethyl) glycine, is a systemic, broad-spectrum herbicide that belongs to the substituted glycine family. Its mechanism of action involves the inhibition of the enzyme 5-enolpyruvylshikimate-3-phosphate synthase, which participates in the metabolic pathway of shikimate (Gomes et al., 2014b). Such enzyme catalyzes the conversion of shikimate-3-phosphate to 5-enolpyruvylshikimate-3-phosphate. This metabolic process leads to the synthesis of the aromatic amino acids phenylalanine, tyrosine, and tryptophan (Alcántara de la Cruz et al., 2016).
Although the mechanism of action of glyphosate is well known, the physiological responses to the herbicide in forest species have been poorly studied. These include inhibition of the photochemical and biochemical stages of photosynthesis, oxidative stress due to accumulation of reactive oxygen species, damage to cell membranes, and alteration in ionic homeostasis (Gomes et al., 2014b).
The physiological responses of the species P. nitens after exposure to the herbicide are unknown. The aim of this work was to evaluate the biochemical and physiological responses of P. nitens to simulated glyphosate drift.
MATERIALS AND METHODS
Trial conditions and plant material: The trial was conducted under greenhouse conditions at the experimental field of the Universidad Nacional de Santiago del Estero, located in El Zanjón, Santiago del Estero, Argentina (27°45'S, 64°18'W). One-year-old P. nitens seedlings were grown in plastic pots containing loam soil and fertilized with N-P-K (20:5:20). Periodic irrigation was carried out to maintain soil moisture content close to field capacity.
Herbicide application: Plants were sprayed with Roundup® Full II (Monsanto Argentina) containing 65% (w/w) of glyphosate potassium salt (N-phosphonomethyl glycine) as the active ingredient in doses of 0, 65 or 130g a.e. ha-1. The herbicide application was carried out according to the conditions described by Meloni & Martínez (2021). Twenty days after herbicide application, leaf samples were taken for chemical determinations.
Determination of concentrations of shikimate: Shikimate was determined according to the method described by Singh & Shaner (1998). One hundred milligrams of leaves were homogenized in 30ml 0,25N HCl. The extract was centrifuged at 25 000g for 15min. A 40µl aliquot of the supernatant was collected to add 0,5ml of periodic acid 1%. After 3h, 0,5mL NaOH 1N and 0,3ml glycine 0,1% were incorporated. The solution was vigorously mixed, and the absorbance was measured at 380nm in a spectrophotometer. Results were expressed in mg g-1 DW.
Quantification of photosynthetic pigments: Leaf samples (0,2g) were ground in a mortar with 80% acetone (v/v); the extract was filtered through glass wool and centrifuged at 15 000 x g for 5min. Supernatant was collected, and absorbances were measured at 663, 647, and 470nm. Concentrations of chlorophyll a, chlorophyll b, and carotenoids were calculated according to Lichtenthaler & Wellburn (1983) equations. Results were expressed in mg g-1 DW.
Determination of mineral composition: Leaves were dried in forced ventilation oven at 60oC to constant weight. They were then ground in a Wiley-type mill, sieved, and digested with 2N HCl. Mineral composition was quantified by inductively coupled plasma atomic emission spectrometry (ICP-AES; PSFO 2.0, Leeman Labs INC., USA), according to the technique described by Chrysargyris et al. (2017).
Experimental design and statistical analysis: A completely randomized experimental design with four replications was used. The experimental unit consisted of one pot containing one P. nitens plant. Results were analyzed with ANOVA and Tukey test.
RESULTS
Doses of 65 and 130g a.e. ha-1 increased concentrations of foliar shikimate by 13% and 28%, respectively, with respect to the untreated control (Fig. 1A). Similarly, concentrations of photosynthetic pigments were very sensitive to the herbicide, decreasing at all tested doses (Fig. 2B, C, D). The lowest concentrations of chlorophyll a, chlorophyll b, and carotenoids were recorded at the dose of 130g a.e. ha-1, with reductions of 62%, 40%, and 50%, respectively, with respect to the control.
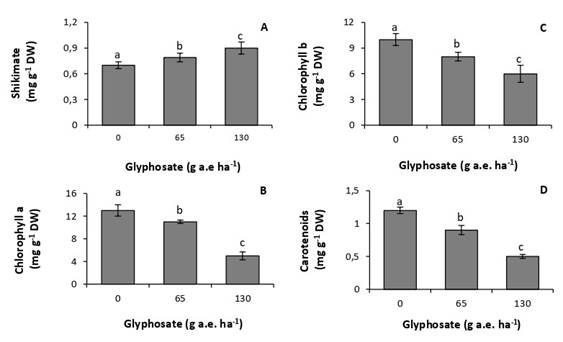
Fig. 1. Concentration of shikimate (A), chlorophyll a (B), chlorophyll b (C), and carotenoids (D) in P. nitens seedlings treated with increasing doses of glyphosate. Values represent the means ± SD of four replicates; when labelled with different letters, they are significantly different (Tukey test; P < 0,05).
Glyphosate affected leaf mineral composition. Concentrations of potassium and magnesium were not affected by the dose of 65g a.e ha-1 (Fig. 2 A, B). In contrast, the dose of 130g a.e. ha-1 produced a 13% and 12,5% reduction in concentrations of potassium and magnesium, respectively. Calcium was the macronutrient most affected by the herbicide, with decreases of 19% and 25% in the treatments of 65 and 130g a.e. ha-1, respectively, with respect to the control (Fig. 2C).
The accumulation of micronutrients was more sensitive to the herbicide than that of macronutrients, decreasing at the two doses tested (Fig. 2D, E, F). The response was proportional to the glyphosate dose. At the dose of 130g a.e. ha-1, concentrations of zinc, manganese, and iron experienced a reduction of 25%, 37,5% and 27%, respectively, with respect to the control.
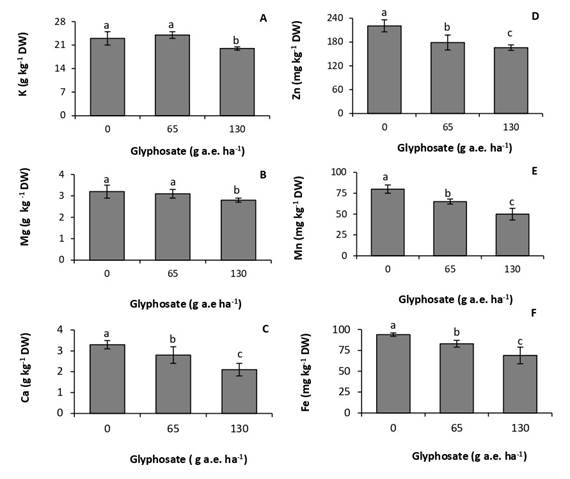
Fig. 2. Concentration of potassium (A), magnesium (B), calcium (C), zinc (D), manganese (E), and iron (F) in P. nitens seedlings treated with increasing doses of glyphosate. Values represent the means ± SD of four replicates; when labelled with different letters, they are significantly different (Tukey test; P < 0,05).
DISCUSSION
At the doses studied, glyphosate affected the physiology and biochemistry of P. nitens. The accumulation of shikimic acid in P. nitens leaves (Fig. 1A) shows that glyphosate inhibited the activity of the enzyme 5-enolpyruvyl-chiquimate-3-phosphate synthase (Schrübber et al., 2014). This result further indicates that the herbicide succeeded in overcoming the leaf epidermal barriers, reaching the interior of the leaves. It also indicates a high sensitivity of the species, since doses as low as 65g a.e ha-1 produced a significant inhibition in enzyme activity.
All glyphosate doses tested produced a decrease in concentrations of photosynthetic pigments (Fig. 1B, C, D). These results coincide with those observed in Zeyheria tuberculosa, a pioneer species native to Brazil, sensitive to glyphosate (de Freitas-Silva et al., 2021). However, in this species the decrease in the concentration of chlorophyll a was observed from a dose of 360g a.e. ha-1, whereas in P. nitens it was observed from a dose of 65g a.e. ha-1. This result shows the high sensitivity of the species to the herbicide. The decrease in concentrations of chlorophyll in plants treated with glyphosate may be due to inhibition in their synthesis or an increase in the rate of degradation (Huang et al., 2012; Gomes & Juneau, 2016). Glyphosate has also been reported to cause damage to chloroplast membranes. This damage includes the loss of proteins associated with the antenna complex of photosystems and, because of it, a decrease in the concentration of pigments (Gomes et al., 2017). It has also been attributed to chloroplast degradation, with concomitant loss of the photosynthetic apparatus (Mateos-Naranjo & Pérez-Martin, 2013).
Decreased concentrations of carotenoids have also been reported in plants of Eugenia uniflora, a native species of the Brazilian Atlantic rainforest (de Freitas-Silva et al., 2021). This response has been attributed to the inhibition of enzymes involved in their synthesis (Sandmann et al., 2006). Carotenoids act as accessory pigments in photosynthesis and as protectors against oxidative stress. This result indicates that glyphosate compromises light absorption and the photochemical stage of photosynthesis. It also shows an increased susceptibility of leaf tissues to the action of reactive oxygen species, which are usually produced under abiotic stress conditions (Meloni & Martinez, 2021).
Glyphosate reduced concentrations of macro- and micronutrients in P. nitens leaves (Fig. 2A, B, C, D, F). Glyphosate inhibits the absorption and transport of cationic macro- and micronutrients due to the formation of poorly soluble glyphosate-metal complexes in plant tissues (Mertens et al., 2018). In agreement with this result, glyphosate reduced concentrations of macro- and micronutrients in two soybean cultivars. The effect was more marked when the herbicide application was made at early stages of the crop (Zobiole et al., 2011). These authors correlated this behavior with the reduction in the concentration of photosynthetic pigments, which in turn was proportional to the photosynthetic rate. Glyphosate can interfere with chlorophyll synthesis by inhibiting magnesium absorption, and the formation of the precursor d-aminolevulinic acid, which requires iron (Gomes et al., 2014b). Metabolic processes taking place in chloroplast are also very sensitive to deficiencies of manganese and zinc (Nilson, 1985).
Biomonitoring consists of the use of species sensitive to pollutants to monitor soil, water, and air quality (Batista et al., 2018). This technique has been proposed as an alternative to evaluate the effect of herbicides in forest ecosystems (Rezende-Silva et al., 2019). For this purpose, variables of photosynthesis (florescence and gas exchange), shikimate accumulation, antioxidants, and malondialdehyde, among others, have been used as biomarkers (Schrübbers et al., 2014; de Freitas-Silva et al., 2020). The high sensitivity of P. nitens to glyphosate suggests the use of this species for this purpose. On the other hand, all the physiological variables studied were sensitive to the herbicide, and thus could be used in biomonitoring works in the region. Among all of them, the response of photosynthetic pigments is of particular interest, since its determination is carried out through simple and low-cost techniques. There are also nondestructive techniques for estimating the concentration of photosynthetic pigments, which are already used for biomonitoring in other regions (de Freitas-Silva et al., 2021).
It is concluded that glyphosate alters the photochemical stage of photosynthesis by decreasing the concentration of photosynthetic pigments. It also interferes with the homeostasis of macro- and micronutrients.
ACKNOWLEDGEMENTS
The authors thank the Consejo de Investigaciones Científicas y Tecnológicas de la Universidad Nacional de Santiago del Estero (CICyT-UNSE) for funding.
ETHICAL, CONFLICT OF INTEREST AND FINANCIAL STATEMENTS
The authors declare that they have fully complied with all pertinent ethical and legal requirements, both during the study and in the production of the manuscript; that there are no conflicts of interest of any kind; that all financial sources are fully and clearly stated in the Acknowledgments section; and that they fully agree with the final edited version of the article. A signed document has been filed in the journal archives.
The declaration of the contribution of each author to the manuscript is as follows: D.A.M.: greenhouse trials, chemical analysis, data processing, and manuscript writing. M.J.N.: field trials and manuscript writing. I.A.: interpretation of results and manuscript writing.
REFERENCES
Alcántara de la Cruz, R., Barro, F., Domínguez-Valenzuela, J.A., & De Prado, R. (2016). Physiological, morphological and biochemical studies of glyphosate tolerance in Mexican Cologania (Cologania broussonetii (Balb.) DC.). Plant Physiology and Biochemistry, 98, 72-80. https://doi.org/10.1016/j.plaphy.2015.11.009
Batista, P.F., Costa, A.C., Megguer, C.A., Lima, J.S., Silva, F.B., Guimarães, D.S., Almeida, G.M., & Nascimento, K.J.T. (2018). Pouteria torta: a native species of the Brazilian Cerrado as a bioindicator of glyphosate action. Brazilian Journal of Biology, 78(2), 296-305. https://doi.org/10.1590/1519-6984.07416
Calzón, M.E., & Giménez, A.M. (2011). Evaluación del potencial dendrocronológico de tipa colorada como herramienta para el manejo forestal en las Yungas de Salta (Argentina). Quebracho Revista de Ciencias Forestales, 19(1-2), 5-13. https://www.redalyc.org/pdf/481/48122207001.pdf
Choumet, J., & Phélinas, P. (2015). Determinants of agricultural land values in Argentina. Ecological Economics, 110, 134-140. https://doi.org/10.1016/j.ecolecon.2014.12.024
Chrysargyris, A., Xylia, P., Botsaris, G., & Tzortzakis, N. (2017). Antioxidant and antibacterial activities, mineral and essential oil composition of spearmint (Mentha spicata L.) affected by the potassium levels. Industrial Crops and Products, 103, 202-212. https://doi.org/10.1016/j.indcrop.2017.04.010
de Freitas-Silva, L., Araújo, T.O., Nunes-Nesi, A., Ribeiro, C., Costa, A.C., & Silva, L.C. (2020) Evaluation of morphological and metabolic responses to glyphosate exposure in two neotropical plant species. Ecological Indicators, 113, 1-11. https://doi.org/10.1016/j.ecolind.2020.106246
de Freitas-Silva, L., Castro, N.D., & Campos da Silva, L. (2021). Morphoanatomical and biochemical changes in Zeyheria tuberculosa exposed to glyphosate drift. Botany, 99(2), 91-98. https://doi.org/10.1139/cjb-2020-0150
Espíndola, Y., Romero, L., Ruiz Diaz, R., & Luna, C. (2018). Influencia de las condiciones de incubación sobre la germinación de semillas de diferentes individuos de Pterogyne nitens. Quebracho Revista de Ciencias Forestales, 26(1), 5-17. https://www.redalyc.org/jatsRepo/481/48160748002/48160748002.pdf
Giménez, A.M., & Moglia, J.G. (2003). Árboles del Chaco Argentino. Guía para el reconocimiento dendrológico. Santiago del Estero el Liberal.
Gomes, MP, Smedbol, E., Carneiro, M., García, Q.S., & Juneau, P. (2014a). Reactive oxygen species and plant hormones. En P. Ahmad (Ed.) Oxidative Damage to Plants (pp. 65-88). Academic Press. https://doi.org/10.1016/B978-0-12-799963-0.00002-2.
Gomes, M.P., Smedbol, E., Chalifour, A., Hénault-Ethier, L., Labrecque, M., Lepage, L., Lucotte, M., & Juneau, P. (2014b). Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid: an overview. Journal of Experimental Botany, 65(17), 4691-4703. DOI:10.1093/jxb/eru269
Gomes, M.P., & Juneau, P. (2016). Oxidative stress in duckweed (Lemna minor L.) induced by glyphosate: is the mitochondrial electron transport chain a target of this herbicide? Environmental Pollution, 218, 402–409. https://doi.org/10.1016/j.envpol.2016.07.019
Gomes, M.P., da Silva F.V., Bicalho, E.M., Borges, F.V., Fonseca, M.B., Juneau, F., & Garcia, Q.S. (2017). Effects of glyphosate acid and the glyphosate-commercial formulation (Roundup) on Dimorphandra wilsonii seed germination: Interference of seed respiratory metabolism. Environmental Pollution, 220(Part A), 452–459. https://doi.org/10.1016/j.envpol.2016.09.087
Huang. J., Silva, E.N., Shen, Z., Jiang, B., & Lu, H. (2012). Effects of glyphosate on photosynthesis, chlorophyll fluorescence and physicochemical properties of cogongrass (Imperata cylindrical L.). Plant Omics Journal, 5(2), 177–183. https://www.pomics.com/lu_5_2_2012_177_183.pdf
Lichtenthaler, H.K., & Wellburn, A.R. (1983). Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions, 11(5), 591-592. https://doi.org/10.1042/bst0110591
Mateos-Naranjo, E., & Perez-Martin, A. (2013). Effects of sublethal glyphosate concentrations on growth and photosynthetic performance of non-target species Bolboschoenus maritimus. Chemosphere, 93(10), 2631–2638. https://doi.org/10.1016/j.chemosphere.2013.09.094
Meloni, D.A., & Martínez, C.A. (2021). Physiological responses of Eucalyptus camaldulensis (Dehnh.) to simulated glyphosate drift. Biofix Scientific Journal, 6(1), 46-53. http://dx.doi.org/10.5380/biofix.v6i1.77236
Mertens, M., Höss, S., Neumann, G., Afzal, J., & Reichenbecher, W. (2018). Glyphosate, a chelating agent-relevant for ecological risk assessment? Environmental Science Pollution Research International, 25(6), 5298-5317. https://doi.org/10.1007/s11356-017-1080-1
Nilsson, G. (1985). Interactions between glyphosate and metals essential for plant growth, In E. Grossbard & D. Atkinson (Eds.), The herbicide glyphosate, (pp. 35–47). Butterworth.
Rezende-Silva, S.L., Costa, A.C., Dyszy, F.H., Batista, P.F., Crispim-Filho, A.J., Nascimento, K.J.T., & Silva, A.A. (2019). Pouteria torta is a remarkable native plant for biomonitoring the glyphosate effects on Cerrado vegetation. Ecological Indicators, 102, 497–506. https://doi.org/10.1016/j.ecolind.2019.03.003
Sandmann, G., Römer, S., & Fraser, P.D. (2006). Understanding carotenoid metabolism as a necessity for genetic engineering of crop plants. Metabolic Engineering, 8(4), 291–302. https://doi.org/10.1016/j.ymben.2006.01.005
Schrübbers, L.C., Valverde, B.E., Sørensen, J.C., & Cedergreen, N. (2014). Glyphosate spray drift in Coffea arabica – Sensitivity of coffee plants and possible use of shikimic acid as a biomarker for glyphosate exposure. Pesticide Biochemistry and Physiology, 115, 15–22. https://doi.org/10.1016/j.pestbp.2014.08.003
Singh, B., & Shaner, D. (1998). Rapid determination of glyphosate injury to plants and identification of glyphosate-resistant plants. Weed Technology, 12(3), 527-530. https://doi.org/10.1017/S0890037X00044250
Zobiole, L.H.S, Kremer, R.J., & Oliveira, R.S. (2011) Constantin, J. Glyphosate affects chlorophyll, nodulation and nutrient accumulation of “second generation” glyphosate-resistant soybean (Glycine max L.), Pesticide Biochemistry and Physiology, 99(1), 53-60. https://doi.org/10.1016/j.pestbp.2010.10.005
