The colours of flowers are a result of pigments produced by plants, which are secondary metabolites. These metabolites have auxiliary functions other than for metabolism and growth of plants (Neilson, Goodger, Woodrow, & Møller, 2013). The synthesis of secondary metabolites by plants has been attributed to survival mechanisms (Akula & Ravishankar, 2011). These metabolites have been shown to address specific needs in the evolution of plants (Pichersky & Gang, 2000; Michael Wink, 2003) and because of the uniqueness of the synthesis of these secondary metabolites in each plant, they are useful as taxonomic markers (Wink & Mohamed, 2003). Furthermore, the age-long chemical warfare between plants and their pests has necessitated the production of these metabolites some of which serve as toxic substances in defense against pathogens and herbivores, and others to aid territory colonization by inhibiting growth of other plants (Wink, 1988). Conversely, some are colourful with aroma and sweetness to attract animals for seed dispersal (Koes, Verweij, & Quattrocchio, 2005).
These plants’ secondary metabolites have been used as raw materials for the production of medicines since prehistory (Cowan, 1999). Particularly useful as antimicrobial agents that have saved many lives. Since 1928 when Alexander Fleming discovered penicillin from mould, antibiotics production has relied heavily on bacterial and fungal sources, with plant sources virtually ignored (Cowan, 1999). In the past few decades however, there has been a rise in number of antimicrobial resistance (AMR) cases reported worldwide (Blair, Webber, Baylay, Ogbolu, & Piddock, 2015) against these antibiotics. The most significant case of antibiotics resistance reported is that to the antibiotics of last resort, colistin (Gao et al., 2016). For this reason, developing new antimicrobial agents is important to stem the growing trend of superbugs spreading across the globe. Promising among the sources of antimicrobial agents is therefore the secondary metabolites from plants (Cowan, 1999). The pigmentation of the secondary metabolites of plants also has usefulness in the textile industry, and those that are halochromic (i.e. change color according to pH changes) are used in colour display and pH indicators (Forster, 1978; Sharifabad & Bahrami, 2016).
In this study, in order to explore the halochromic properties and the antimicrobial potentials of the crude extracts of several ornamental plants, we examined both in flowering plants in the gardens around International Institute of Tropical Agriculture, Benin Republic. The characteristic shape of the petals of the flowers were used to identify the selected flowers. Crude extracts from the petals were tested against common pathogens of rice and the halochromic properties of each flower established.
MATERIALS & METHODS
Sample collection: the flower samples used in this experiment were collected from the garden at International Institute of Tropical Agriculture (IITA), Cotonou, Benin. The bacteria pathogens were provided and earlier characterized by the Pathology Unit of Africa Rice, Cotonou (Lee, Hong, & Kim, 2010; Afolabi et al., 2016; Kini et al., 2017).
Identification of flowers: The characteristic shape of leaf, number of petals and colour were used to identify the flowers by comparing them with images of documented flowers.
Extraction of pigments from flower petals: Pigments were extracted from the petals of all the flowers except in E. crassipes, where the thick, glossy leaves were used. Pigments were extracted by crushing 1g of petals in 1mL of solvent with the use of a mortar and pestle. The slurry generated were transferred into 1,5mL Eppendorf tubes and vortexed (Fisher Vortex Genie 2TM– from Fisher Scientific) for 10mins for maximum extraction of the pigments. The resultant mixtures were then centrifuged (Eppendorf Centrifuge Model no 5415D) at 11 000RPM for 30mins to recover the solvents, with the supernatant collected into fresh Eppendorf tubes using a micropipette and stored at 4˚C.
Investigating the halochromic properties of the flower extracts: In order to establish the halochromic properties of the extracts from the flowers, aqueous solutions with pH ranging from 2–12 were prepared by titrating sodium hydroxide with hydrochloric acid. pH of the titrations was monitored with a pH meter and titration stopped at each desired pH points. Transparent 96-well plates were used as colour plates. Seven wells for the pH range and five columns for the different flowers were used. Each well contained 20µL of flower extract, with 20µL of each of the pH range solutions added to the wells on a row. Colour change of the extracts in each well was observed and recorded.
Antimicrobial screening: This screening was carried out in order to investigate the antibacterial property and rate of bacteria inhibition of the crude extracts of the flowers using two techniques respectively: Agar well diffusion method and growth inhibition in liquid culture, Luria Broth (LB).
Agar Well Diffusion Method: Lysogeny broth solidified with agar was prepared using, 2g of peptone (BactoTM Peptone), 2g of NaCl (Sodium Chloride Certified ACS Crystalline from Fisher Scientific), 1g of yeast extract (BactoTM Yeast Extract from Becton Dickinson microbiology systems) and 3g of agar (DifcoTMAgar, granulated solidifying agent from Becton Dickinson microbiology systems) in 200mL distilled water and then autoclaved (Napco® Model 8000-DSE Autoclave). About 20mL of the autoclaved nutrient agar was then poured into petri dishes and allowed to solidify. The bacterial samples were applied to the plates using top agar gel. Briefly, 2% agar was made in distilled water and then autoclaved. Secondary culture of the bacterial samples were inoculated at 1:100 in LB medium and allowed to grow to mid log phase. The 2% agar solution was allowed to cool down to 40oC over water bath and the bacterial growth at mid log phase was quickly added at 1:1 and spread on the nutrient agar plates. A 2mm sterile cork borer was used to punch wells 2cm apart on the nutrient agar plates. The wells were labelled and filled with 80µL of the crude extracts. Known penicillin concentrations, autoclaved distilled water and acetonitrile were used as positive and negative controls respectively. The plates were then incubated at 37oC overnight after the crude extracts have diffused into the plates.
Bacterial growth rate assay: Luria Broth (LB) medium was prepared using 2g of peptone, 2g of NaCl and 1g of yeast extract in 200mL of distilled H2O. The mixture was split into five aliquots, 500mL each in conical flasks to hold 40mL of the medium and autoclaved. Secondary culture of the bacterial samples was inoculated at 1:100 in LB medium and allowed to grow to mid log phase. Thereafter, 40 µL of penicillin (100mg/mL) and 40µL of flower plant extracts (T. erecta) were added to two of the five labelled flasks respectively. Then, 200µl of the bacteria strain was added to the two conical flasks with the antibiotic and flower extract. A third flask was also inoculated as control. The three flasks were mounted on a shaker (Stuart Orbital Shaker SSL1) at 27˚C and 200RPM. The starting OD600 was recorded and the growth rate monitored at time interval of 30min at OD600 using a spectrophotometer (Jenway 6 300 Spectrophotometer).
Ethical, conflict of interest and financial statements: the authors declare that they have fully complied with all pertinent ethical and legal requirements, both during the study and in the production of the manuscript; that there are no conflicts of interest of any kind; that all financial sources are fully and clearly stated in the acknowledgements section; and that they fully agree with the final edited version of the article. A signed document has been filed in the journal archives.
RESULTS
Identification of the flowery plants: The brightly colored petals and leaves of the flowers enabled easy identification when compared with documented flowers. The Fig. 1 shows the images of the flowers collected at IITA Benin’s garden and their corresponding names based on the physical characteristic of the leaves and petals.
Extraction of the pigments with aqueous and organic solvents: The pigments in the various petals of the selected ornamental plants exhibited different solubility in water, methanol and acetonitrile. For each flower, the most compatible solvent on the basis of level of solubility and retention of original colour, was selected as shown in Table 1.
Halochromic properties of the crude extracts in pH range of 2-12: the halochromic properties of the various crude extracts from the flowers were examined at pH range of 2–2. The Fig. 2 shows that T. erecta and A. blanchetii extracts have distinct halochromic properties.
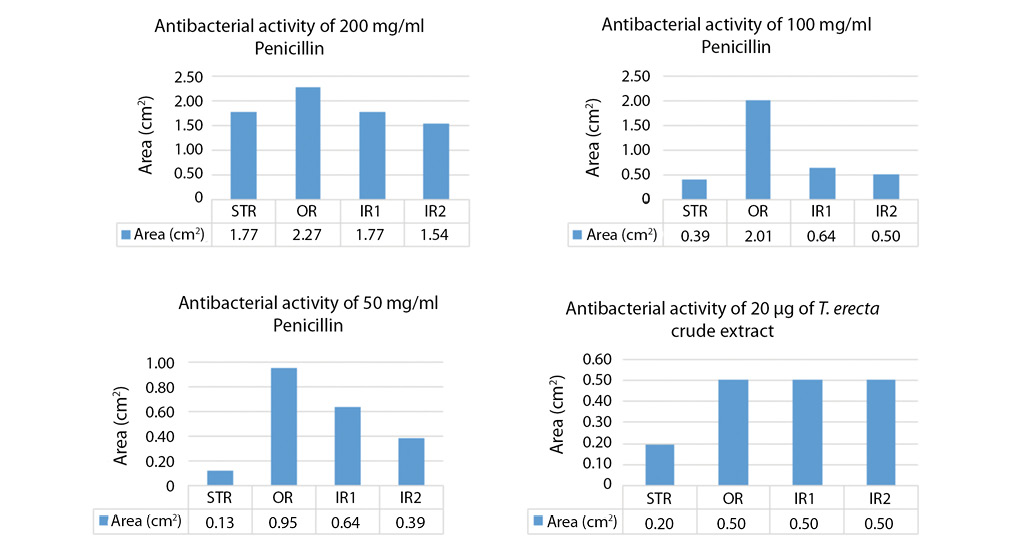
Top agar diffusion screening of the antimicrobial activity of the extracts on Gram-negative bacterium, Pantoea agglomerans: the antibacterial activities of the extracts were examined on P. agglomerans and a purified crystalline penicillin at three concentrations of 200mg/mL, 100mg/mL and 50mg/mL were used as positive control while sterile distilled water was used as negative control. The Fig. 3 shows the top agar plates of the incubation and bacterial growth inhibitions by the extracts and controls observed at 6hrs and 18hrs. Out of all the crude extracts only T. erecta shows antibacterial activities against the P. agglomerans. Penicillin also inhibited the growth of the gram-negative bacterium as shown in Fig. 3.
We, however, observed resistance being developed by the bacterium after 18hrs of incubation and a noticeable resistance ring forming against penicillin. Fig. 3b shows the various ring formations and the inhibition rings represented as Bar Chat in Fig. 4. The highest concentration of penicillin inhibited the growth of the bacterium to 2,27cm2 in 24hrs but the bacterium resisted by reducing the inhibition to 1,77cm2 forming the first inner ring and then to 1,54cm2 forming the second inner ring. No resistance rings were formed with the growth inhibition by T. erecta.
Comparison of the extracts on gram-positive Bascillus subtilis and gram-negative bacterium Xanthomonas oryzae pv. oryzae using top agar diffusion: all the extracts have some levels of inhibition against the gram-positive bacterium except C. thevetia. However, only T. erecta and A. blanchetii have antimicrobial activities against X. oryzea as shown in Fig. 5.
We further examined the ability of the T. erecta in bacterial inhibition growth in LB medium culture comparing it with penicillin.
DISCUSSION
Halochromic properties of the crude extracts: purple pigmentation in flowers are associated with the synthesis of anthocyanins by plants (Archetti et al., 2009). Anthocyanins are a subclass of phenolic phytochemicals in the forms of anthocyanidin glycosides and acylated anthocyanins (Khoo et al., 2017). These are water soluble secondary metabolites (Geissman, 1955; Khoo et al., 2017) and their colour depend on the pH of the solution because of the ionic charge of their molecular structure (Turturica, Oancea ,Râpeanu, & Bahrim, 2015). They have been found in all the tissues of higher plants and are derivatives of anthocyanidins (Andersen & Jordheim, 2010). Halochromic property of anthocyanidins have been proposed to be a good pH indicator (Michaelis, Schubert, & Smythe, 1936) and antimicrobial activities established in berries (Miceli et al., 2009; Genskowsky et al., 2016). The halochromic property of anthocyanin extracted from raspberry juice is being used as colour indicator to monitor the pH change of wound which depends on healing stages and presence of infection (Coomber, 2018). The exhibition of halochromic property observed in T. erecta and A. banchetti indicated they have pigments with ionic molecular structure. Further work will be required to confirm the identity of these charged pigments.
Antimicrobial activity: Thunbergia erecta is one of the common species of Thunbergia (Sultana, Chatterjee, Roy, & Chandra, 2015). Members of these species have been found to have antimicrobial activities against both gram positive and gram negative bacteria (Jeeva, Johnson, Aparna, & Irudayaraj, 2011; Jenifer, et al., 2014; Kosai, Jiraungkoorskul, & Jiraungkoorskul, 2015). The anti-microbial activities of T. erecta against Panteoa agglomeran and Xanthomonas oryzae are remarkable. Pantoea agglomerans and Xanthomonas oryzae pv. oryzae are both pathogens of rice cultivation (Salzberg et al., 2008; Lang et al., 2010; Lee et al., 2010) . They are rod-shaped, gram-negative bacteria found in both temperate and tropical parts of the world and cause 10-50% yield losses. Recently, Pantoea agglomerans were discovered to be the plight of farmers on wild rice Oryza longistaminata plants near Tanguiéta town, at Pendjari National Park, northwest Benin republic (Afolabi et al., 2016; Kini et al., 2017).
The mode of action of antimicrobial agents are classified into six: cell wall synthesis inhibition, protein synthesis inhibition, DNA synthesis inhibition, RNA synthesis inhibition, mycolic acid synthesis inhibition and folic acid inhibition (Hugo, 1967; Hahn, 2012) . Penicillin is a cell wall inhibitor (Wise & Park, 1965). Antimicrobial resistance of bacteria to penicillin is the production of enzymes capable of destroying the b-lactam ring of penicillin (Tenover, 2006). Once the penicillin is destroyed, resistance ring will form signifying clearance of the antibiotics and demonstrating the resistance status of the bacteria. The additional outer membrane layer of gram-negative organisms differentiates them from gram-positive organisms. This provides additional protection and thereby enhancing survival during stress conditions (Schwechheimer & Kuehn, 2015). The Fig. 3 and 5 show the gram-negative P. agglomerans and X. oryzae respectively resisting some of the flower extracts while the gram-positive, B. subtilis, was susceptible to all the extracts except C. thevetia.
Based on the results obtained from this research, it can be concluded that the secondary metabolites from the crude extract of T.erecta is a promising antimicrobial candidate whose bioactive component can be exploited to aid in the fight against the rising problem of antimicrobial resistance. The absence of resistance ring by bacteria against T. erecta shows that the mode of action of this antimicrobial agent is different from that of penicillin. Isolation, purification and identification of the pigments from the crude extract of T.erecta would give better understanding of the active pigment responsible for these useful properties and add to the arsenal of molecules to fight antimicrobial resistance.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Rousseau Djouaka, AgroEcoHealth IITA and his team for allowing us to use his facilities at IITA Benin Republic for this experiment. We also acknowledge other members of the Pathology unit of AfricaRice for their contributions.
REFERENCES
Afolabi, O., Amoussa, R., Bilé, M., Oludare, A., Gbogbo, V., Poulin, L., . . . Silué, D. (2016). First report of bacterial leaf blight of rice caused by Xanthomonas oryzae pv. oryzae in Benin. Plant Disease, 100(2), 515-515. DOI:10.1094/PDIS-07-15-0821-PDN
Akula, R., & Ravishankar, G. A. (2011). Influence of abiotic stress signals on secondary metabolites in plants. Plant signaling & behavior, 6(11), 1720-1731. DOI:10.4161/psb.6.11.17613
Andersen, Ø. M., & Jordheim, M. (2010). Anthocyanins. eLS. Chichester: JohnWiley & Sons DOI:10.1002/9780470015902.a0001909.pub2
Archetti, M., Döring, T. F., Hagen, S. B., Hughes, N. M., Leather, S. R., Lee, D. W., . . . Schaberg, P. G. (2009). Unravelling the evolution of autumn colours: an interdisciplinary approach. Trends in ecology & evolution, 24(3), 166-173. DOI:10.1016/j.tree.2008.10.006
Blair, J. M., Webber, M. A., Baylay, A. J., Ogbolu, D. O., & Piddock, L. J. (2015). Molecular mechanisms of antibiotic resistance. Nature Reviews Microbiology, 13(1), 42. DOI:10.1038/nrmicro3380
Coomber, A. (2018). Wound dressing materials incorporating anthocyanins derived from fruit or vegetable sources: US Patent App. 15/830,188. Washington, DC: U.S. Patent and Trademark Office.
Cowan, M. M. (1999). Plant products as antimicrobial agents. Clinical microbiology reviews, 12(4), 564-582. DOI:10.1128/CMR.12.4.564
Forster, M. (1978). Plant pigments as acid-base indicators-An exercise for the junior high school. Journal of Chemical Education, 55(2), 107. DOI:10.1021/ed055p107
Gao, R., Hu, Y., Li, Z., Sun, J., Wang, Q., Lin, J., . . . Li, D. (2016). Dissemination and mechanism for the MCR-1 colistin resistance. PLoS pathogens, 12(11), e1005957. DOI:10.1371/journal.ppat.1005957
Geissman, T. (1955). Anthocyanins, chalcones, aurones, flavones and related water-soluble plant pigments Moderne Methoden der Pflanzenanalyse/Modern Methods of Plant Analysis (pp. 450-498): Springer. DOI:10.1007/978-3-642-64958-5_12
Genskowsky, E., Puente, L. A., Pérez‐Álvarez, J. A., Fernández‐López, J., Muñoz, L. A., & Viuda‐Martos, M. (2016). Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensi s (Molina) Stuntz] a Chilean blackberry. Journal of the Science of Food and Agriculture, 96(12), 4235-4242. DOI:10.1002/jsfa.7628
Hahn, F. E. (2012). Mechanism of action of antibacterial agents. Berlin, Germany: Springer Science & Business Media.
Hugo, W. (1967). The mode of action of antibacterial agents. Journal of Applied Microbiology, 30(1), 17-50.
Jeeva, S., Johnson, M., Aparna, J., & Irudayaraj, V. (2011). Preliminary phytochemical and antibacterial studies on flowers of selected medicinal plants. International Journal of Medicinal and Aromatic Plants, 1(2), 107-114.
Jenifer, S., Priya, S., Laveena, D. K., Singh, S. J. S., & Jeyasree, J. (2014). Sensitivity patterns of some flowering plants against Salmonella typhi and Pseudomonas aeruginosa. Journal of Pharmaceutical Sciences, 3, 1212-1220.
Khoo, H. E., Azlan, A., Tang, S. T., & Lim, S. M. (2017). Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food & nutrition research, 61(1), 1361779. DOIi:10.1080/16546628.2017.1361779
Kini, K., Agnimonhan, R., Afolabi, O., Milan, B., Soglonou, B., Gbogbo, V., . . . Silué, D. (2017). First report of a new bacterial leaf blight of rice caused by Pantoea ananatis and Pantoea stewartii in Benin. Plant Disease, 101(1), 242-242. DOI:10.1094/PDIS-06-16-0939-PDN
Koes, R., Verweij, W., & Quattrocchio, F. (2005). Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends in plant science, 10(5), 236-242. DOI:10.1016/j.tplants.2005.03.002
Kosai, P., Jiraungkoorskul, K., & Jiraungkoorskul, W. (2015). Review of antidiabetic activity of “Rang Jeud” Thunbergia laurifolia. Journal of Applied Pharmaceutical Science, 5, 99-103.
Lang, J. M., Hamilton, J. P., Diaz, M. G. Q., Van Sluys, M. A., Burgos, M. R. G., Vera Cruz, C. M., . . . Leach, J. E. (2010). Genomics-based diagnostic marker development for Xanthomonas oryzae pv. oryzae and X. oryzae pv. oryzicola. Plant Disease, 94(3), 311-319. DOI:10.1094/PDIS-94-3-0311
Lee, H., Hong, J., & Kim, S. (2010). First report of leaf blight caused by Pantoea agglomerans on rice in Korea. Plant Disease, 94(11), 1372-1372. DOI:10.1094/PDIS-05-10-0374
Miceli, N., Trovato, A., Dugo, P., Cacciola, F., Donato, P., Marino, A., . . . Taviano, M. F. (2009). Comparative analysis of flavonoid profile, antioxidant and antimicrobial activity of the berries of Juniperus communis L. var. communis and Juniperus communis L. var. saxatilis Pall. from Turkey. Journal of Agricultural and Food Chemistry, 57(15), 6570-6577. DOI:10.1021/jf9012295
Michaelis, L., Schubert, M. P., & Smythe, C. (1936). Potentiometric study of the flavins. Journal of Biological Chemistry, 116, 587-607.
Neilson, E. H., Goodger, J. Q., Woodrow, I. E., & Møller, B. L. (2013). Plant chemical defense: at what cost? Trends in plant science, 18(5), 250-258. DOI:10.1016/j.tplants.2013.01.001
Pichersky, E., & Gang, D. R. (2000). Genetics and biochemistry of secondary metabolites in plants: an evolutionary perspective. Trends in plant science, 5(10), 439-445. DOI:10.1016/S1360-1385(00)01741-6
Salzberg, S. L., Sommer, D. D., Schatz, M. C., Phillippy, A. M., Rabinowicz, P. D., Tsuge, S., . . . Kelley, D. (2008). Genome sequence and rapid evolution of the rice pathogen Xanthomonas oryzae pv. oryzae PXO99 A. BMC genomics, 9(1), 204. DOI:10.1186/1471-2164-9-204
Schwechheimer, C., & Kuehn, M. J. (2015). Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nature Reviews Microbiology, 13(10), 605. DOI:10.1038/nrmicro3525
Sharifabad, A. N., & Bahrami, S. H. (2016). Halochromic Chemosensor From Poly (acrylonitrile)/Phenolphthalein Nanofibers as pH Sensor. IEEE Sensors Journal, 16(4), 873-880. DOI:10.1109/JSEN.2015.2495338
Sultana, K., Chatterjee, S., Roy, A., & Chandra, I. (2015). An Overview on Ethnopharmacological and Phytochemical properties of Thunbergia sp. Med Aromat Plants, 4(217), 2167-0412.100021.
Tenover, F. C. (2006). Mechanisms of antimicrobial resistance in bacteria. American journal of infection control, 34(5), S3-S10. DOI:10.1016/j.ajic.2006.05.219
Tuturica, M., Oancea, A., Râpeanu, G., & Bahrim, G. (2015). Anthocyanins: naturally occuring fruit pigments with functional properties. Annals of the University Dunarea de Jos of Galati Fascicle VI--Food Technology, 39(1), 9-24,
Wink, M. (1988). Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theoretical and applied genetics, 75(2), 225-233. DOI:10.1007/BF00303957
Wink, M. (2003). Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry, 64(1), 3-19. DOI:10.1016/S0031-9422(03)00300-5
Wink, M., & Mohamed, G. I. (2003). Evolution of chemical defense traits in the Leguminosae: mapping of distribution patterns of secondary metabolites on a molecular phylogeny inferred from nucleotide sequences of the rbcL gene. Biochemical Systematics and Ecology, 31(8), 897-917. DOI:10.1016/S0305-1978(03)00085-1
Wise, E. M., & Park, J. T. (1965). Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proceedings of the National Academy of Sciences, 54(1), 75-81. DOI:10.1073/pnas.54.1.75
https://orcid.org/0000-0002-0447-0398
https://orcid.org/0000-0003-0798-8843
https://orcid.org/0000-0001-8657-7166
https://orcid.org/0000-0001-7363-2576
https://orcid.org/0000-0003-2877-1483