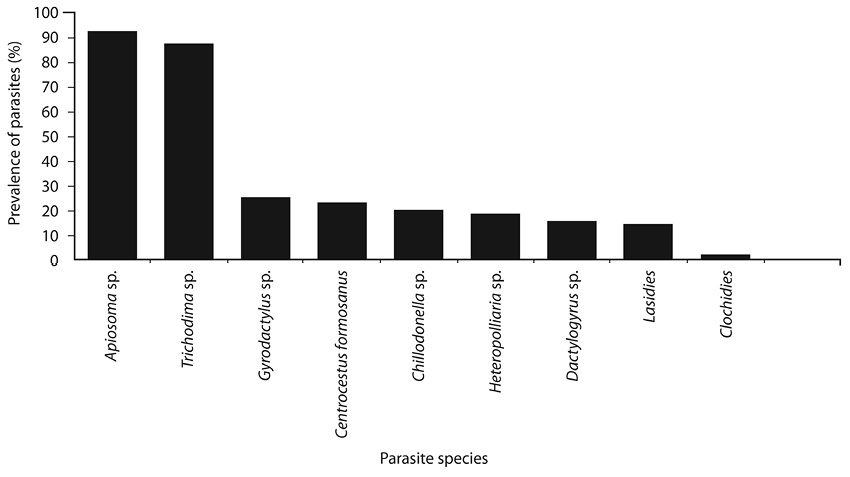
Fig. 1. Prevalence of parasites from Nile tilapia larvae during the period of study.
Parasites of Nile Tilapia larvae Oreochromis niloticus (Pisces: Cichlidae)
in concrete ponds in Guanacaste, Northern Costa Rica
Donald Arguedas C.1, Cesar Ortega S. 2, Simón Martínez C.2 & Ángel Astroza C.3
1. LARED Laboratory, National Technical University, Guanacaste, Costa Rica; darguedas@utn.ac.cr
2. Center for Research and Advanced Studies in Animal Health, Faculty of Veterinary Medicine and Animal Science, Autonomous University of the State of Mexico, Toluca, State of Mexico, Mexico; cortegas@uaemex.mx
3. Laboratory of Molecular and Cellular Biology, Department of Biology and Chemistry, San Sebastián University, Chile, AV. General Lagos 1163 Valdivia, Chile; angel.astroza@uss.cl
Received 06-vi-2017 • Corrected 03-viii-2017 • Accepted 24-viii-2017
ABSTRACT: Tilapia is the second most important cultured species in the world fish culture but it can be affected by parasites. We conducted a cross-sectional parasitic study in tilapia larvae during sexual reversion for two seasons in Costa Rica. A total of 320 larvae from a concrete pond were necropsied and we found ten parasite species: Ichtyobodo sp., Apiosoma sp., Chillodonella sp., Heteropollaria sp., Trichodina sp., Dactylogyrus sp., Girodactylus sp., Centrocestus sp., lasidies and glochidies (two larval forms). These were classified in five taxonomic groups (two subtypes of protozoa, two metazoan classes and a type of mollusk). Protozoans and monogeneans (except Trichodina sp.) had a higher prevalence in the rainy season, when water had more solid waste, while digeneans and molluscs were more prevalent in the dry season, with different infection dynamics over gills, skin, fins and head.
Key words: Prevalence; parasites; Nile tilapia; sex reversal process; Costa Rica.
Interest on tilapia species cultivation began in Africa and Asia due to the low cost and high nutritional value of these animals, which were beneficial to feed the human population in these developing areas (Food and Agriculture Organization of the United Nations, 2007). Currently, tilapia is the second most important group of fish for the world fish culture. In 2014, it reached a production volume from 200 000 tones contributing China approximately 35% and Taiwan and Indonesia, being the main exporters of whole product and frozen fillet, while the trade in fresh fillets in Latin America has been led by Guatemala, Costa Rica and Honduras (Food and Agriculture Organization of the United Nations, 2015).
Tilapia is considered relatively resistant to diseases (Coward & Bromage, 2000), but it can be affected by different parasites. There are reports about protozoa presence as: Ichthyophthirius multifiliis, Trichodina, Chilodonella, Ichtyobodo necatrix; Monogenean: Cichlidogyrus, Gyrodactylus, Dactylogyrus; Nematodes: Contracaecum sp. and parasitic crustaceans such as: Argulus, Ergasitus and Lernea (Basson, Van-As & Paperna, 1983; Kazubski, 1986; Okaeme, Obiekezie, Lehman, Antai & Madu, 1988; Paperna 1991; Noga & Flower, 1995; Pariselle & Euzet, 1996; Tavares, Martins & Moraes, 2001; Martins, Marchiori, Nunes, & Rodrigues, 2010; Pariselle, Nyom & Bilong, 2013). Although, the greatest affection is recorded in fingerlings and larvae.
Some epidemiological research of parasites in tilapia have established that infections are increased in intensive culture systems because of high fish density affecting directly the population growth of parasite species (Garcia, Osorio-Saraiba & Constatino, 1993).
In different situations, if the parasites do not reach the level or amount necessary to cause an epizootic, they can weaken the host fish, or be the entry route for other infections. (Tesana, Thabsripair, Suwannatrai, Haruay, Piratae, Khampoosa, & Jones, 2014).
Some tilapia parasites are also important for public health (Sommerville, 1982; Tesana et al., 2014). For example, Clonorchis sinensis causes hepatomegalias, cachexia, ascites and cirrhosis. O. viverrini is the causative agent of obstructive jaundice, pancreatitis, cholangitis, and cholangiocarcinoma (Choi et al., 2006). Moreover, C. formosanus has been reported causing diarrhea, epigastric pain and indigestion (Chai et al., 2013).
In Costa Rica the cultivation of Nile tilapia is the main productive freshwater activity. In recent years it has experienced an industrial development, with annual productions exceeding the 20 000 MT (Metric Tonnes). The major companies are located in Guanacaste province (Costa Rican Institute of Fisheries and Aquaculture, 2014).
Despite the economic and cultural importance of tilapia for Costa Rica, studies that show the parasitic infectious agents dynamics that affect tilapia under production systems, are lacking. A digenean trematode Centrocestus formosanus was reported as the cause of death of tilapia fingerlings in the dry Pacific of Costa Rica (Arguedas, Dolz, Romero, Jiménez & León, 2010) determining experimentally part of helminth life cycle; although, there are no similar studies in Costa Rica. About wild fish, exists only an investigation (Sandlund, Daverdin, Choudhury, Brooks & Diserud, 2010), but findings remained at the project report level.
The present study aims to register parasites in Nile tilapia larvae during the process of sexual reversion.
MATERIALS AND METHODS
Study area: The research was carried out between January and December of 2014, in an aquaculture farm located in San Miguel, Cañas, Guanacaste, Costa Rica (10º20’00” N- 85º05’00”W), between 50 and 60m above sea level, with an average temperature of 27,9ºC and 2 266mm precipitation, in a tropical dry forest (Janzen, 1983).
Sampling fish: A total number of 320 tilapia larvae of approximately 0,85g of weight in process of sexual reversion (SR) were captured during the investigation of a concrete pond from 300m2 (20m x 25m), 160 fish were caught in the month of April (dry season) and another 160 fish in October (rainy season). The sample size for the calculation of prevalence for each of the samples was made using EPIINFO software, version 6.0 (Dean, Dean, Burton, & Dicker, 1995), with a prevalence of 50%, an absolute error of 10% and 95% confidence level. The larvae were transferred separately in plastic bags provided with oxygen to the Laboratory for Analysis of Wastewater and Diseases Freshwater of the National Technical University, Cañas, Guanacaste, Costa Rica. Immediately, fish were examined for parasites under light microscope, and parasites did not identified, were referred to the Center for Research and Advanced Studies in Animal Health (CIESA), Faculty of Veterinary Medicine and Animal Science, Autonomous University of the State of Mexico, Toluca, State of Mexico, Mexico.
Parasitological examination: The 320 larvae were examined to observe parasites and microscopic alterations in body surface. The eyes, gills, skin and fins were analyzed under microscope at 40-100X. Infested fish were examined. Immediately they were into glass petri box physiological serum added to 0.65%. Moreover, parasites were picked up using forceps and Pasteur pipettes by the methodology recommended by Noga (2011). The found parasites were classified taxonomically (Paperna, 1996).
Statistic analysis: The prevalence of parasites was analyzed as follows:
The prevalence (%) of the parasites was estimated as the ratio between the number of infected fish and the number of examined fish expressed in percentages.
The Chi square test (χ2) was used to determine the degree of dependence between prevalence of the species found and sampling seasons. The differences were statistically significant if the p value was ≤ p 0,05.
Physicochemical parameters: Dissolved oxygen, pH and water temperature were measured with an YSI 85® multiparameter oxygenator and turbidity with the Secchi disk. Measurements were performed in triplicate (8, 13 and 16 hours) for 21 days (period of the sexual reversal process). The values were averaged each season.
RESULTS
Taxonomic groups: Ten species of parasites were recorded in five taxonomic groups: two protozoan subtypes, two metazoan types and one mollusk type. 1. Sarcomastigophora: (Ichtyobodo sp.)=Costia; 2. Ciliophora: (Apiosoma sp.)=Glosatella sp., Chillodonella sp., (Heteropollaria sp.)=Epistylis sp., Trichodinas; 3. Monogeneans: (Dactylogyrus sp. and Girodactylus sp.); 4. Digeneans: (Centrocestus sp.); 5. Molluscs (two larval forms, lasidies and glochidies).
Parasites prevalence between seasons: The parasite prevalence in dry and rainy seasons are showed in table 1. Ichtyobodo sp. had a prevalence on gills of 15% in dry season and 84% in rainy season, while, Apiosoma sp. with prevalence level of 84% and 100% respectively. The prevalences were significant in rainy season for both parasites species (χ2 =11, 59, p≤0, 05).
Comparing Ichtyobodo sp and Apiosoma sp. prevalence identified from skin, Ichtyobodo sp registered 9% in dry season and 29% in rainy season, while Apiosoma sp. with prevalence level of 27% and 84% respectively. The prevalence were not statistically significant (χ2 =0, 0063, p>0, 05).
In dry season Chillodonella sp. showed a prevalence level of 3% but higher in rainy season (61%). However, this parasite was not registered on skin any season at all.
Heteropollaria sp. was not found into gills, however, it was a protozoan with a lower prevalence on skin (3%) registered in dry season, compared with a 56% in rainy season.
Trichodina genus was not identified to level species. These ciliates were recovered from gills and skin. During dry season was registered a prevalence on gills of 82% and 53% in rainy season. The skin prevalence peaked in dry season (87%) when was compared with rainy season (57%), although, the prevalence were not statistically significant any season.
The two monogeneans recovered were Dactylogyrus sp. and Girodactylus sp. Dactylogyrus was found only into gills (5%) in dry season and rainy season 45% of prevalence. Contrary, Girodactylus sp was only registered on skin showing a higher prevalence level in rainy season (78%) than dry season (3%).
The unique digenean attached was Centrocestus sp. (metacercariae). In dry season this zoonotic trematode was registered from different locations (60% into gills, 1% on skin and 1% on fins). In contrast, in rainy season only was into gills (12%).
With regard to parasite molluscs, in both seasons were found lasidies and glochidies fixed from gills and fins. The lasidies prevalence were less than 5%, but 25% and 15% on head in dry and rainy seasons, respectively. Larvae of glochidies type were found in gills and fins, but their prevalence were less than 5% in both seasons too.
Parasites prevalence between species: The prevalence by species are shown in Fig. 1. Apiosoma sp. and Trichodina sp. showed the highest prevalence (92% and 87% respectively). While, lowest prevalence were registered by lasidies (14%) and glochidies (2%).
Physicochemical parameters of water: Table 2 shows the physicochemical parameters of water. The water temperature (ºC) was higher in dry season; likewise, the dissolved oxygen concentration, with a value of 4,4 mg/L, whereas, in rainy season was registered a value of 2mg/L. Turbidity had a value of 28cm, being highest in the rainy season.
DISCUSSION
In Costa Rica there are few studies to know the parasitic biodiversity that affects tilapia fish (Arguedas et al., 2010). This study is the first report for identification of parasite diversity on tilapia fish larvae under systems culture, in stage reversal sexual process, in which parasitic agents can cause high mortalities, or immunosuppressive (Conroy, 2005; Pádua, Ishikawa, Ventura, Martins, & Tavares, 2013).
In this research we detected 10 species of parasites, into 5 taxonomic groups, however, in another study in fish cultured in Brazil, 16 species were identified in four groups (Pantoja, Neves, Montagner, & Tavares-Dias, 2012). On the other hand, the calculated prevalence by wild fish (Sandlund et al., 2010) were lower than those recorded in our study, but they registered 50 species, although, the report was from 27 sites with different environments.
In regards protozoa, prevalence recorded for Ichtyobodo sp. are higher than prevalence reported by Williams & Jones (1994) who researched parasite biodiversity of cichlids. Paperna (1996) found Ichtyobodo necator (Costia necatrix) affecting juvenile cichlids. In our study, Ichtyobodo sp. the etiological agent of costiasis disease was found attached from gills and skin, hypothesizing to tilapia as a host of this protozoan, which could be dispersed around Costa Rica, through fingerlings sending among farms. Ichtyobodo necator, is a cichlid´s parasite reported in other fish families, producing high mortality in culture farm with different water temperatures (Deen, Hady, Kenawy, & Mona, 2015). This find agreed with our report of prevalence parasite in dry and rainy seasons, caused by temperature water differences.
The Apiosoma sp. prevalence, also reported as (Glosatella sp.) were increasing from dry season to rainy season, on gills and skin. According to Noga (2000) this ciliate had high prevalence in waste-water solids. This information agrees with our outcomes for the turbidity measurement obtained on the rainy season, reaching a 28 cm value.
The higher Chillodonella sp. prevalence in rainy season is similar to reported in Ciprinus carpius culture (Roberts, 1981) a serious epizootic problem during rainy months in freshwater fish (Monir et al., 2015). Moreover, it is correlated with hyperinfections from cultured tilapia in lowest temperature water to 22°C (Kazubski, 1986; Abdel-Baki & Quraishy, 2014), similar to our investigation. However, our findings showed to Chillodonella sp. was attached to the gills, in contrast with reported in several previous studies (Oldewage & Van As, 1987), who observed the parasite on skin tilapia hybrids.
Heteropollaria sp. (Epistylis genus) showed prevalence differences between seasons (Martins, Cardoso, Marchiori, & Benites, 2015), showing high values in rainy season, which agrees with our report. Probably, this protozoa has an optimal reproduction at lower temperature with hypoxic water conditions.
Trichodina affecting gills and skin, which confirms the adaptation degree of these protozoa (Basson & Vas As, 1989). The Trichodina high prevalence on fish in sex inversion phase could be associated to its simple reproduction cycle (Madsen, Buchmann, & Mellergaard, 2000), caused by limited water exchange and high fish number into concrete pond resulting a massive colonization. The Trichodina prevalence were different between seasons. In dry season were higher than rainy season, in contrast with study reported by Abd & Hassan (1999) who found high prevalence on winter from tilapia farms located in Saudi Arabia. We agree with Conroy (2005) who reports trichodines as fastidious parasites in tilapia sex inversion phase on fresh and salty water. We observed trichodines have opportunistic conditions due to high prevalence even under stress (critical values of water quality) registered in rainy season.
Dactylogyrus is a monogenean attached in gills, contrary Gyrodactylus usually on skin (Paperna, 1991; Williams & Jones, 1994). This information supports our results that both worms can be collected from gills and skin in the same fish. Moreover, these species have been associated with heavy infections under stress of negative water quality, in consonance with this research. These evidences suggest an extensive physiological adaptation to different environment factors. Another possible explanation is Dactylogyrus develops eggs in dry season, given birth in rainy season.
Centrocestus formosanus was an identified trematode found in dry season infecting gills, skin and fins. For this parasite difference of prevalence between seasons were similar to reported on O. niloticus cultured during flooding season in Vietnam farms (Thien, Dalsgaard, Thanh, Olsen, & Murrell, 2007), but minor to the trematodes diversity reported by Wiriya, Clausen, Inpankaew, Thaenkham, Jittapalapong, Satapornvanit, & Dalsgaard (2013) that determined in Nile tilapia to Stellantchasmus falcatus, Haplorchis pumilio and Procerovum varium. The findings about C. formosanus are of interest for health authorities, particularly because it is a new report for this zoonotic parasite in Costa Rica.
Studies to know the pathological impact of these parasites in Costa Rican aquacultural farms are necessary. Moreover, the spread and impact in wild fish populations.
In the present study, we revealed 10 species of parasites. Ichtyobodo sp, Apiosoma sp, Chillodonella sp, Heteropollaria sp, Trichodina sp, Dactylogyrus sp, Girodactylus sp, Centrocestus sp, lasidies and glochidies (two larval forms). The results indicate higher prevalence for protozoans and monogeneans in rainy season, while digeneans and molluscs are more prevalent in dry season, having different infection dynamics over gills, skin, fins and head.
The information obtained may provide strategies in aquaculture management to reduce potential economic losses in fry tilapia production caused by parasitic infection. It is important to check annually parasite population in Nile tilapia farms.
ACKNOWLEDGEMENTS
This project was funded by the National Technical University and was possible thanks to specific sponsorship from Costa Rican Aquaculture and Fisheries Law N°8436.
REFERENCES
Abdel-Baki, A. A. S., & Al-Quraishy, S. (2014). First record of Chilodonella spp. (Ciliophora: Chilodonellidae) in cultured Nile tilapia (Oreochromis niloticus) in the central region of Saudi Arabia. Pak Journal Zoology, 46(3), 657-660. Retrieved from http://zsp.com.pk/pdf46/657-660%20_9_%20PJZ-1676-14%2019-5-14.pdf
Abd, M., & Hassan, A. (1999). Trichodiniasis in Farmed Freshwater Tilapia in Eastern Saudi Arabia. Marine Science, 10(1), 157-168. doi:10.4197/mar.10-1.11
Arguedas, D., Dolz, G., Romero, J. J., Jiménez, A. E., & León, D. (2010). Centrocestus formosanus (Opisthorchiida: Heterophyidae) como causa de muerte de alevines de tilapia gris Oreochromis niloticus (Perciforme: Cichlidae) en el Pacífico seco de Costa Rica. Revista de Biología Tropical, 58(4), 1453-1465.doi:10.15517/rbt.v58i4.5423
Basson, L., & Van As, J. G. (1989). Differential diagnosis of the genera in the family Trichodinidae (Ciliophora: Peritrichida) with the description of a new genus ectoparasitic on freshwater fish from southern Africa. Systematic parasitology, 13(2), 153-160. doi: 10.1007/bf00015224
Basson, L., Van As, J. G., & Paperna, I. (1983). Trichodinid ectoparasites of cichlid and cyprinid fishes in South Africa and Israel. Systematic parasitology, 5(4), 245-257. doi:10.1007/bf00009159
Chai, J. Y., Sohn, W. M., Yong, T. S., Eom, K. S., Min, D. Y., Lee, M. Y., ... & Rim, H. J. (2013). Centrocestus formosanus (Heterophyidae): human infections and the infection source in Lao PDR. Journal of Parasitology, 99(3), 531-536. doi:10.1645/12-37.1
Choi, D., Lim, J. H., Lee, K. T., Lee, J. K., Choi, S. H., Heo, J. S., ... & Hong, S. T. (2006). Cholangiocarcinoma and Clonorchis sinensis infection: a case-control study in Korea. Journal of hepatology, 44(6), 1066-1073 doi:10.1016/j.jhep.2005.11.040
Conroy, G. (2005). Importantes enfermedades detectadas en tilapias cultivadas en América Central y del Sur. Primera Jornada Acuícola. San José, Costa Rica.
Costa Rican Institute of Fisheries and Aquaculture. (Setiembre de 2014). Desarrollo del Procesamiento y de la Comercialización de la Tilapia Producida en las Grandes Cuencas Latinoamericanas. En L. Dobles (Presidencia), Simposio de acuacultura. Simposio llevado a cabo en el Congreso de Acuacultura, San José, Costa Rica.
Coward, K., & Bromage, N. R. (2000). Reproductive physiology of female tilapia broodstock. Reviews in Fish Biology and Fisheries, 10(1), 1-25. doi.org/10.1023/A:1008942318272
Dean, A. G., Dean, J. A., Burton, A. H., & Dicker, R. C. (1995). Epi Info [computer program]. Version 604c.
De Pádua, S. B., Ishikawa, M. M., Ventura, A. S., Jerônimo, G. T., Martins, M. L., & Tavares, L. E. R. (2013). Brazilian catfish parasitized by Epistylis sp. (Ciliophora, Epistylididae), with description of parasite intensity score. Parasitology research, 112(1), 443-446. doi:10.1007/s00436-012-3069-5
Deen, N., Abd El Hady, O. K., Kenawy, A. M., & Mona, S. Z. (2015). Study of prevailing external parasitic diseases in cultured freshwater tilapia Oreochromis niloticus Egypt. Life Science Journal, 12(8), 30-37. doi:10.7537/marslsj120815.06
Food and Agriculture Organization of the United Nations. (2014). GLOBEFISH - Analysis and information on world fish trade. Retrieved from http://www.fao.org/in-action/globefish/market-reports/resource-detail/en/c/358402/
Food and Agriculture Organization of the United Nations. (2007). Informe de la multiplicación de la tilapia en América. Retrieved from http://www.fao.org/home/es/
García, L. J., Sarabia, D. O., & Constantino, F. (1993). Prevalencia de los parásitos y las alteraciones histológicas que producen a las tilapias de la laguna de Amela, Tecomán, Colima. Veterinaria México, 24(3), 199-205.
Janzen, D. H. (1983). Costa Rican natural history. Chicago, ON: The University of Chicago Press.
Kazubski, S. L. (1986). The Trichodinid Ciliates from fish, Tilapia sp. from Lake Victoria (Kenya) and description of Trichodina equatorialis nom. Acta parasitológica, 25(1), 445-448.
Kobayashi, J., Vannachone, B., Sato, Y., Manivong, K., Nambanya, S., & Inthakone, S. (2000). An epidemiological study on Opisthorchis viverrini infection in Lao villages. Southeast Asian J Trop Med Public Health, 31(1),128-32. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11023079?report=abstract
Madsen, H. C., Buchmann, K., & Mellergaard, S. (2000). Trichodina sp. (Ciliophora: Peritrichida) in eel Anguilla anguilla in recirculation systems in Denmark: host-parasite relations. Diseases of aquatic organisms, 42(2), 149-152. doi:10.3354/dao042049
Martins, M. L., Cardoso, L., Marchiori, N., & Benites de Pádua, S. (2015). Protozoan infections in farmed fish from Brazil: diagnosis and pathogenesis. Revista Brasileira de Parasitologia Veterinária, 24(1), 1-20. doi:10.1590/s1984-29612015013
Martins, M. L., Marchiori, N., Nunes, G., & Rodrigues, M. P. (2010). First record of Trichodina heterodentata (Ciliophora: Trichodinidae) from channel catfish, Ictalurus punctatus cultivated in Brazil. Brazilian Journal of Biology, 70(3), 637-644. doi:10.1590/s1519-69842010000300022
Monir, S., Bagum, N., Rahman, S., Ashaf-Ud-Doulah. M., Bhadra, A., & Borty, S.C (2015). Parasitic diseases and estimation of loss due to infestation of parasites in Indian major carp culture ponds in Bangladesh. International Journal Fisheries and Aquatic Studies, 2(5), 118-122. Retrieved from http://www.fisheriesjournal.com/archives/2015/vol2issue5/PartC/2-5-45.pdf
Noga, E. J. (2000). Fish disease: diagnosis and treatment. Iowa, ON: Acribia.
Noga, E. J., & Flowers, J. R. (1995). Invasion of Tilapia mossambica (Cichlidae) viscera by the monogenean Enterogyrus cichlidarum. The Journal of parasitology, 81(5), 815-817.
Oldewage, W. H., & Van As, J. G. (1987). Parasites and winter mortalities of Oreochromis mossambicus. South African Journal of Wildlife Research, 17(1), 7-12. Retrieved from http://journals.co.za/docserver/fulltext/wild/17/1/3208.pdf?expires=1502997994&id=id&accname=guest&checksum=F70DD96449A9CF1D0C0E7BA227D96DB0
Okaeme, A. N., Obiekezie, A. I., Lehman, J., Antai, E. E., & Madu, C. T. (1988). Parasites and diseases of cultured fish of Lake Kainji area, Nigeria. Journal of fish biology, 32(3), 479-481.doi:
Paperna, I. (1996). Parasites, infections and diseases of fishes in Africa: An update. CIFA Technical Paper (FAO). No. 31. Retrieved from http://www.fao.org/docrep/008/v9551e/V9551E00.HTM
Paperna, I. (1991). Diseases caused by parasites in the aquaculture of warm water fish. Annual Review of Fish Diseases, 1(1), 155-194. doi:10.1016/0959-8030(91)90028-i
Pariselle, A., & Euzet, L. (1996). Cichlidogyrus Paperna, 1960 (Monogenea, Ancyrocephalidae): gill parasites from West African Cichlidae of the subgenus Coptodon Regan, 1920 (Pisces), with descriptions of six new species. Systematic Parasitology, 34(1), 109-124. doi:10.1007/bf00009685
Pariselle, A., Nyom, A. R. B., & Bilong, C. F. (2013). Checklist of the ancyrocephalids (Monogenea) parasitizing Tilapia species in Cameroon, with the description of three new species. Zootaxa, 3599(1), 78-86. doi:10.11646/zootaxa.3599.1.7
Sandlund, O. T., Daverdin, R., Choudhury, A., Brooks, D. R., & Diserud, O. H. (2010). A survey of freshwater fishes and their macroparasites in the Guanacaste Conservation Area (ACG), Costa Rica. NINA repport. Retrieved from https://brage.bibsys.no/xmlui/handle/11250/2441242
Roberts, R. J. (2012). Fish pathology. Idaho, ON: John Wiley & Sons.
Sommerville, C. (1982). The life history of Haplorchis pumilio (Looss, 1896) from cultured tilapias. Journal of Fish Diseases, 5(3), 233-241. doi:
Tavares-Dias, M., Martins, M. L., & Moraes, F. R. (2001). Fauna parasitária de peixes oriundos de “pesque-pague” do município de Franca, São Paulo, Brasil. I. Protozoários. Revista Brasileira de Zoologia, 18(1), 67-79. doi:10.4025/actascibiolsci.v28i3.253
Tesana, S., Thabsripair, P., Suwannatrai, A., Haruay, S., Piratae, S., Khampoosa, P., ... & Jones, M. K. (2014). Parasite surveys and environmental management for prevention of parasitic infection in cultivated Barbonymus gonionotus (Cyprinidae) in fishponds, in an opisthorchiasis endemic area of northeast Thailand. Aquaculture, 428, 54-60. doi:10.1016/j.aquaculture.2014.02.031
Villalobos-Rojas, F., Herrera-Correal, J., Garita-Alvarado, C. A., Clarke, T., & Beita-Jiménez, A. (2014). Actividades pesqueras dependientes de la ictiofauna en el Pacífico Norte de Costa Rica. Rev. Biol. Trop, 62(4), 119-138. doi:10.15517/rbt.v62i4.20038
Pantoja, M. F., Neves, L., Montagner, D., & Tavares-Dias, M. (2012). Protozoan and metazoan parasites of Nile tilapia Oreochromis niloticus cultured in Brazil. Revista MVZ Córdoba, 17(1), 2812-2819. Retrieved from http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0122-02682012000100002
Thien, P. C., Dalsgaard, A., Thanh, B. N., Olsen, A., & Murrell, K. D. (2007). Prevalence of fishborne zoonotic parasites in important cultured fish species in the Mekong Delta, Vietnam. Parasitology research, 101(5), 1277-1284. doi:10.1007/s00436-007-0633-5
Williams, H. H., Jones, A., & Crompton, D. W. T. (1994). Parasitic worms of fish. London, ON: Taylor & Francis.
Wiriya, B., Clausen, J. H., Inpankaew, T., Thaenkham, U., Jittapalapong, S., Satapornvanit, K., & Dalsgaard, A. (2013). Fish-borne trematodes in cultured Nile tilapia (Oreochromis niloticus) and wild-caught fish from Thailand. Veterinary parasitology, 198(1), 230-234. doi:10.1016/j.vetpar.2013.08.008
RESUMEN: Parásitos de larvas de tilapia del Nilo Oreochromis niloticus (Pisces: Cichlidae) en estanques en Guanacaste, Costa Rica. La tilapia es la segunda especie de cultivo más importante en el mundo de la pesca, pero puede verse afectada por parásitos. Realizamos un estudio transversal de parásitos en larvas de tilapia durante la reversión sexual en dos temporadas en Costa Rica. Un total de 320 larvas de un estanque de concreto fueron necropsiados y encontramos diez especies de parásitos: Ichtyobodo sp., Apiosoma sp., Chillodonella sp., Heteropollaria sp., Trichodina sp., Dactylogyrus sp., Girodactylus sp., Centrocestus sp., lasidios y gloquidios (dos formas larvarias). Estos se clasifican en cinco grupos taxonómicos (dos subtipos de protozoarios, dos clases de metazoarios y un tipo de molusco). Los protozoarios y monogeneos (excepto Trichodina sp.) tuvieron una mayor prevalencia en la época lluviosa, cuando el agua tenía más residuos sólidos, mientras que los digeneos y moluscos eran más frecuentes en la estación seca, con diferentes dinámicas de infección sobre las branquias, la piel, las aletas y la cabeza.
Palabras clave: Prevalencia; parásitos; tilapia del Nilo; proceso de reversión sexual; Costa Rica.
Prevalence = Number of host species infested with a particular parasite species ×100
Total number of hosts examined.
TABLE 1
Parasite prevalence in tilapia larvae organs from
a concrete pond of 200m2 during reversal sex process
in the dry and rainy season.
|
Parasites |
Location |
Prevalence (%) |
|
|
Dry Season |
Rainy Season |
||
|
Ichtyobodo sp. (Costia) |
Gill Skin |
15 9 |
84 29 |
|
Apiosoma sp. (Glossatella) |
Gill Skin |
84 27 |
100 84 |
|
Chillodonella sp. |
Gill Skin |
3 0 |
61 0 |
|
Heteropollaria sp. (Epistilys sp.) |
Gill Skin |
0 3 |
0 56 |
|
Trichodina sp. |
Gill Skin |
82 87 |
53 57 |
|
Dactylogyrus sp. |
Gill Skin |
5 0 |
45 0 |
|
Gyrodactylus sp. |
Gill Skin |
0 3 |
0 78 |
|
Centrocestus sp. |
Gill Skin Fin |
60 1 1 |
12 0 0 |
|
Lasidies |
Gill Fin Head |
0 3 25 |
1 2 15 |
|
Glochidies |
Gill Fin |
1 1 |
2 3 |
Fig. 1. Prevalence of parasites from Nile tilapia larvae during the period of study.

TABLE 2
Physicochemical parameters of water from
a concrete pond of 200m2 in the dry and rainy season
during reversal sex process.
|
Parameter |
Dry season (Mean) |
Rainy season (Mean) |
|
Dissolved oxygen (mg/L) |
4,4 |
2 |
|
pH |
7,6 |
4,8 |
|
Temperature (ºC) |
31 |
21 |
|
Turbidity(cm) |
28 |
55 |