In the Caspian Sea, three kilka species including common (Clupeonella caspia Svetovidov, 1941), anchovy (C. engrauliformis Borodin, 1904) and bigeye kilka (C. grimmi Kessler, 1877) are found. These three pelagic species are the primary planktivores in the Caspian Sea (Mamedov, 2006) and are prey species for key predators (Pourgholam et al., 1996), so, these species are important in ecosystem function of the Caspian sea and are important to the economies of coastal areas.
Bigeye kilka can be distinguished from other kilkas by their bigger eye and greater number of vertebra (Berg, 1948). This species is distributed in the central and southern Caspian and extends farther from shore than the two other kilka species. It is found primarily at depths greater than 50-70 m with low light. This species is less tolerant to high temperature and salinity than anchovy and common kilka (Prikhodko, 1981). Bigeye kilka can spawn throughout the year, but most spawning occurs between November and April in the southern Caspian Sea (Fazli et al., 2004; Aliasghari et al., 2011).
Kilka fishing is conducted during nighttime using under-water light and funnel nets. Recently catch composition of kilkas has changed and now common kilka is dominant in the catch (Karimzadeh et al., 2010). Kilka stocks have been decreasing, which threatens jobs and industries with the potential for subsequent social and economic problems (Esmaeili Sari et al., 2002). Potential causes of the decline of kilka populations include invasion of ctenophore Mnemiopsis leidyi into the Caspian Sea (Ivanov et al., 2000), kilka population changes (Esmaeili Sari et al., 2002, Daskalov and Mamedov, 2007, Roohi et al., 2008), environmental changes, pollution, and overfishing (Paritskii et al., 2001, Fazli, 2011).
Although there have been studies of bigeye kilka biology (Fazli et al., 2004; Karimzadeh et al., 2010; Aliasghari et al., 2011) and stock assessment (Pourgholam et al., 1996; Fazli and Rouhi, 2001; Fazli, 2011) in the southern Caspian Sea, there is little attention has been paid to changes in the bigeye kilka population over time in the southern Caspian. The aim of the present study was to determine the population structure, mortality, survival, and exploitation rate of C. grimmi in southern waters of the Caspian Sea in order to assess the current status of the population and variation during recent years for stock management and constant exploitation of kilkas via optimum fishing management.
MATHERIALS AND METHODS
Sampling area: The sampling areas were in Iranian waters of the southern Caspian in Mazandaran province with the vessels of Babolsar Harbor (Fig. 1) in 52° 55” E and 36° 51”N. These vessels were equipped with cone nets and underwater electric lights. This research was conducted during a two-year period from January 2009 to December 2010 at depths of 70 to 100 meters and 2027 samples were collected. Species identification was aided using Macroscopic and morphologic characteristics key (Berg, 1948; Svetovidov, 1963).
Sample analysis: In the laboratory, fork length (mm) was measured with a measuring board and body weight was measured using digital balance (nearest 0.1 g).
For age determination, sagittal otoliths of fish were used. Otoliths were put in glycerin and age was determined using a stereomicroscope with a top-projected light and a black background (Francis&Campana, 2004).
The length-weight relationship was calculated as following:
W = a Lb
where W is the fish weight (g), L is the fork length (mm), a is constant and b is the regression trend line slope (Bagenal, 1978).
Determining the growth pattern via t-test, the b amount was evaluated using the following formula (Morey et al., 2003):
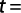

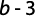
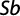
where b is the regression trend line slope and Sb is the standard deviation of b.
Von Bertalanffy growth parameters were calculated using a non-linear estimation method (Pauly et al., 1992) as:
Lt = L∞ [1-exp -k(t-t0)]
where t is age in years, Lt is fish length at age t, t0 is length at 0, L∞ is L-infinity and k is the growth coefficient.
Natural mortality coefficient (M) was estimated from Pauly’s equation (Pauly, 1999).
log(M) = -0.0066 - 0.279log(L∞) + 0.6543log(K) + 0.4634log(T)
where T is the annual average water temperature of fish habitat which was estimated 12OC in a former study (Karimzadeh et al., 2010).
Survival rate (S) was calculated using the catch curve method (Ricker, 1975). Total mortality (Z) was transformed from the survival rate as:
Z = – ln S
Total mortality (Z) is sum of the two sources of mortality (M and F) together (Prakarn, 2002); so, the coefficient of fishing mortality (F) was calculated as the coefficient of total mortality (Z) minus the coefficient of natural mortality (M) using the equation:
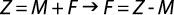
Exploitation rate was calculated from the fishing mortality divided by the total mortality as (Sparre & Venema, 1989):
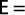

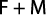
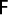
Statistical analysis software: The software applications Microsoft Office Excel 2003, SPSS 10.0, and FISAT were used for statistical analysis.
RESULTS
During 2009, the total catch of common kilka, anchovy, and bigeye kilka was 20,741 tons (5121 VN), of which common kilka had the highest catch proportion (88.3%). The proportion of anchovy and bigeye kilka were 7.2% and 4.5%, respectively. In 2010, total kilka catch was 21,216 tons (5544 VN), of which common kilka had the highest catch (86.2%), followed by anchovy (9.6%) and big eye kilka (4.2%).
Average fork lengths of bigeye kilka in 2009 and 2010 were 120.06±11.95 and 119.12±13.42 mm and average weights were 14.91±5.28 and 15.17±5.24 g, respectively (Table 1).
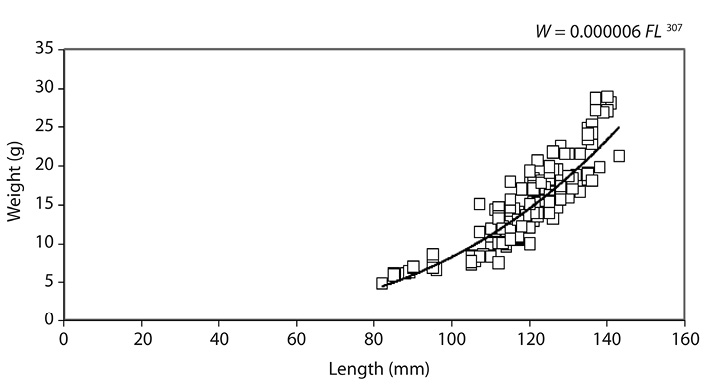
The fork length and body weight relationship in bigeye Kilka (2010) was W = 0.000006 E 3.07 and the regression correlation was R2=0.834 (Fig. 2). The calculated b was significantly more than 3 and t-test showed the positive allometric growth pattern (p<0.05).
Age composition of bigeye kilka catch included 6 age groups, 2-7 years (both females and males). Age 3 was the largest age group, composing 34.82% and 33.56% of the catch in 2009 and 2010, respectively. Average age during these two years was 3.97±0.88 and 3.90±1.22 yr, respectively.
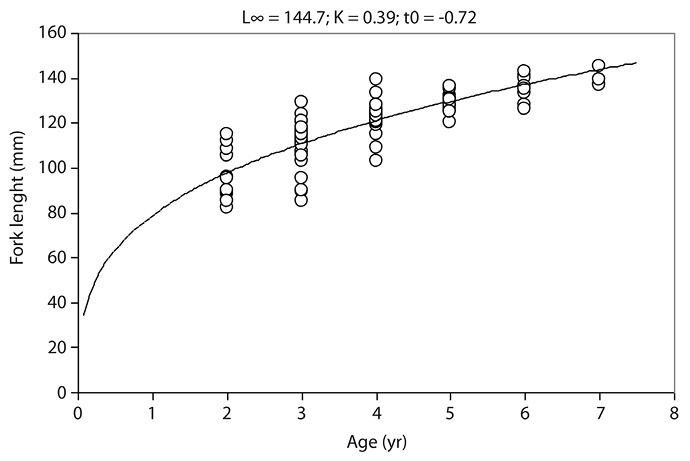
Von Bertalanffy growth parameters were similar both years with no significant difference. In 2009, bigeye kilka parameters were: L∞=145.1mm, k=0.37yr-1, t0=-0.63yr with the growth equation: Lt = 145.1[1-exp -0.37(t+0.63)]. Parameters in 2010 were: L∞=144.7mm, k=0.39yr-1, t0=-0.72yr (Lt = 144.7[1-exp -0.39(t+0.72)]) (Fig. 3).
The coefficient of natural mortality (M) in 2009 and 2010 was 0.448yr-1 and 0.419 yr-1, respectively. Using catch curves, the annual survival rate (S) was estimated 0.305 yr-1 in 2009 and 0.309 yr-1 in 2010. According to the survival rate, the instantaneous coefficient of total mortality (Z) in 2009 and 2010 was estimated 1.184 yr-1 and 1.174 yr-1, respectively. Instantaneous coefficients of fishing mortality (F) were 0.736 yr-1 and 0.775 yr-1 in 2009 and 2010, respectively. In these years, the exploitation rate (E) of bigeye kilka was estimated 0.621 and 0.660, respectively.
DISCUSSION
Studies of fish population dynamics are essential for understanding human and natural effects on fish populations and for devising effective management plans. Pelagic species are vulnerable to environmental variations; thus, environmental factors should be considered in stock assessment (Zhang and Lee, 2001). During the past years, the Caspian Sea environment has changed significantly in response to impacts of human and natural factors such as pollution, fluctuations of sea water level as well as invasive species (Salmanov, 1999; Ivanov, 2000; Rouhi et al., 2008).
Bigeye catch proportion in 1996 was 22.1% of total kilka catch (Pourgholam et al., 1996), in 1997 was 12.6% afterwards decreased to 6.2% in 2001 (Fazli et al., 2002) then reached 4.2% in 2010. Decreasing process in bigeye catch amount can be due to over fishing or increasing life depth (out of reach for fishing vessels) because of the pressure of natural factors such as M. leidyi as well as two other kilka species on bigeye niche.
Average weight and fork length of bigeye kilka in southern Caspian increased continuously from 1997 to 2008 (Fazli et al., 2009; Karimzadeh et al., 2010); afterwards average weight increased continuously but fork length had an inconsiderable decline in 2009 continuing to 2010. Relative increasing size in catch is perhaps because of over fishing, decreasing the reproduction rate or standardization of net meshes. On the other hand, increasing average weight simultaneous with decreasing length shows relatively good feeding sources for this species in high depth.
Calculated b in present study was as b>3 indicating this species has a positive allometric growth pattern (p<0.05) and agree with the previous studies in which b was more than 3 (Belyaeva et al., 1989, Fazli et al., 2004, Mamedov, 2006; Karimzadeh et al., 2010). Reported b amounts show that bigeye kilka has relative good environmental and feeding conditions perhaps because of its deep life area which is less affected by human and natural factors.
Age study showed that 4-years old fish were dominant in bigeye kilka population in 2001 (Fazli et al., 2009). Afterwards, age 3 and 4 had the most catch proportion in 2008 (Karimzadeh et al., 2010) and in present study age 3 was dominant. Dropping process of average catch age can be due to the bigeye habitat affected by natural factors and environmental changes. Considering the standardization of kilka fishing net mesh, decreasing the average age of bigeye kilka in recent years can show the increasing growth rate which caused this species to be catched in lower age. Considering the growth coefficient of slow-growing species k ≤ 0.1 (Jennings et al., 2001), k amount in bigeye kilka is more than this given, so this species is a fast-growing aquatic. Study on k shows that this coefficient decreased from 1989 to 2004 (Belyaeva et al., 1989; Fazli, 2007) but increased again to 2010. High k amounts can reflect the high natural mortality (Holt, 1965); in other words, fast-growing species will tolerate high natural mortality. According to Gulland (1969), species with high M amount have high k amount as: 1≤M/k≤2. On the other hand, L∞ for bigeye has increased from 1989 (Belyaeva et al., 1989) to 2004 (Mamedov, 2006), until present study. Increased L∞ amount in 2010 in comparison with 1989 indicates this species tries to decline the natural mortality because bigger fish will have fewer predators (Sparre & Venema, 1998); in other words, natural mortality has an inverse relation with L∞. Changes in k and L∞ of bigeye in recent years indicates that its habitat has been affected by the Caspian Sea ecological changes due to the ctenophore M. leidyi and also the pressure of two other kilka species to bigeye habitat. Bigeye Kilka with feeding on its own feed sources in deeper waters tries perhaps compensating the pressure of natural competitors by enlarging as a way against predators.
Natural mortality of bigeye kilka has decreased since 1995 to 2001 (Fazli et al., 2009) till 2009 and 2010 in present study; inversely the fishing mortality had an increasing process during this period. Decreasing M in recent years can be resulted of changes in life depth of bigeye kilka to deeper waters as well as enlarging the body size; and increasing F perhaps is due to recent increasing fishing depth by vessels (from 40-50m to more about 100m in depth) and approaching bigeye habitat in depth more than 70 m (Prikhodko, 1981). On the other hand, over fishing is an adverse pressure on this species. Using standard fishing gear, timely seasonal limitation of fishing and reducing the catch amount in fishing program can decrease the fishing mortality. The exploitation rate (E) of bigeye kilka in 2009 and 2010 was more than the desirable amount (E≈0.5) suggested by Gulland (1983) as the theoretical exploitation rate that could maximize the harvest. Thus, bigeye kilka stock was under over-fishing. There is a direct relation between the exploitation rate and fishing mortality (Beverton&Holt, 1959); thus, the increasing of both exploitation rate and fishing mortality occurs simultaneously.
It is noticeable that the increasing catch of common kilka was simultaneous with decreasing the proportion of two other kilka species, anchovy and bigeye, in catch composition (Fazli, 2011). These can be resulted of human and natural factors such as over-fishing and the invader ctenophore M. leidyi which are the main causes for decreasing kilka biomass in southeastern Caspian. In fact, the most likely primary cause of decreased kilka stock is the invasion and spread of M. leidyi in Caspian (Daskalov and Mamedov, 2007). This ctenophore transported with ballast water from the Black Sea and appeared in the Caspian Sea in 1999 (Ivanov et al., 2000) and feeds on zooplankton, fish eggs and larvae (Kideys and Moghim, 2003). M. leidyi has a high reproduction rate because of suitable environmental conditions; so, it can be potential threat for the Caspian Sea habitants (Esmaeili Sari et al., 2002).
During the last decade, kilka fishing in Mazandaran waters (southern Caspian) was conducted in depth more than 40 m (Fazli and Rouhi, 2001); but now in deeper waters more than 70 m up to 100 m; indicating changes in kilka life depth as well as fishing depth. During previous years, bigeye kilka has not significantly been affected by the Caspian Sea pelagic changes due to inhabiting in deep regions (Aliasghari et al., 2011); but the adverse competition on feeding and ecological niche between M. leidyi and kilka, specially common and anchovy kilka, can lead to unilateral intercept with the ctenophore dominance (Esmaeili Sari et al., 2002) which will certainly influence bigeye population. Penetrating common kilka to depth more than 30-40 m, will dislodge anchovy kilka population from its habitat (Fazli et al., 2005). As a result of common kilka force, anchovy kilka population went to deeper parts of the sea and make pressure on bigeye population. Moreover, feeding competition can be one reason of the stock decrease of pelagic fishes such as kilka in the Caspian Sea. Continuing this process, no recovery could be expected in pelagic fishery (Finenko et al., 2006). Thus, monitoring vertical movements of kilka population, effecting factors and changes of its niche and feeding sources, M. leidyi spread specially in water column and controlling it with biological methods is essential for constant catch of Kilka in Caspian.
This study showed that bigeye kilka has affected by human and natural factors, M. leidyi and over fishing. In recent years, the Caspian Sea ecosystem and kilka population incurred damage because of invader ctenophore. Moreover, the exploitation rate of bigeye kilka is more than the acceptable level. Regarding to the importance of kilka fishes in food chain and human usage, continuing such process leads to destruction of the kilka population and thereupon the Caspian ecosystem. So, it is necessary to exert a hard surveillance on the Caspian Sea in the case of M. leidyi controlling and kilka fishing programming.
ACKNOWLEDGEMENTS
I am grateful to Farrokh Parafkandeh for specimen collection; Saber Vatandoust and Shayan Ghobadi for their help and support.
REFERENCES
Aliasghari, M., Parafkandeh Haghighi, F., & Karimzadeh, G. (2011). Survey on reproduction and growth parameters of bigeye kilka Clupeonella grimmi Kessler, 1877 in southern parts of the Caspian Sea. Abstract Book of International Congress on Applied Biology. 1-2 Sep. 2011, Mashhad, Iran. p. 80.
Bagenal, T. B. (1978). Methods of assessment of fish production in freshwater. Blackwell scientific publication. 365p.
Belyaeva, V. N., Kazancheev, E. N., & Raspopov, V. M. (1989). The Caspian Sea: Ichthyofauna and commercial resources. Moscow, Nauka. 236 p.
Berg, I. S. (1948). Fresh water fishes of Iran and Adjacent countries, trade Zoologicheskogo Institute Academy Nauk USSR. pp. 783-858.
Beverton, R. J. H., & Holt, S. J. (1959). A review of life spans and natural mortality rates of fish in nature and their relation to growth and other physiological characteristics. In: eds. G.E.W. Wolstenholme and M. O’Connor. CIBA Choice, dynamics and uncertainly. Chapman and Hall, N.Y. USA, 570p.
Esamaeili Sari, A., Farshchi, P., & Darvishi, F. (2002). Study of feeding competition between the invader ctenophore Mnemiopsis leidyi and anchovy kilka in southern coasts waters of the Caspian Sea. Journal of Marine Sciences and Technologies. 1: 25-42 (In Persian).
Daskalov, G.M., & Mamedov, E.V. (2007). Integrated fisheries assessment and possible causes for the collapse of anchovy kilka in the Caspian Sea. ICES Journal of Marine Science, 64: 503-511.
Fazli, H. (2007). Population Dynamics and Stock Assessment of Kilka (Genus: Clupeonella) in Iranian Waters of the Caspian Sea. Phd thesis, Pukyong National University, Department of Marine Biology, 125p. Available on: http://kosfic.chonnam.ac.kr
Fazli, H. (2011). Some environmental factors effects on species composition, catch and CPUE of kilkas in the Caspian Sea. International Journal of Natural Resources and Marine Sciences. 1: 157-164.
Fazli, H., & Rouhi, A. (2001). Probable effects of Mnemiopsis leidyi enterance on species composition, catch and stock of kilka fishes in southern Caspian Sea (during 1997-2001). Iranian Scientific Journal of Fisheries, 1: 63-72.
Fazli, H., Bourani, M., Janbaz, A., & Rouhi, A. (2002). Kilka fishing and anchovy kilka biological characteristics before and after Mnemiopsis Leidyi in the Caspian Sea. 1st Natinal Congress on the Caspian Sea Ctenophores, Iran, Sari, 2002. 16 p. (In Persian).
Fazli H., Sayyad Bourani M., & Janbaz, A. A. (2004). Assessing the biological characteristics of Clupeonella grimmi in Iranian commercial catch in the Caspian Sea. Iranian Scientific Fisheries Journal, 13: 125-138.
Fazli, H., Sayyad Bourani, M., & Janbaz, A. A. (2005). Common kilka (Clupeonella cultriventris caspia) biological characteristics and the effects of Mnemiopsis Leidyi in southern part of the Caspian Sea. Researching and Making Journal (aquatics), 69: 87-96.
Fazli, H., Zhang, C.I., Hay, D.E., Lee, C.W. (2009). Fishery Biological Characteristics and changes in biomass of bigeye kilka (Clupeonella grimmi) in the Caspian Sea. Asian Fisheries Science, 22: 943-960.
Finenko, G., Kideys, A., Anninsky, B., Shiganova, T., Roohi, A., Tabari, R., Rostami, H., & Bagheri, S. (2006). Invasive ctenophore Mnemiopsis leidyi in the Caspian Sea: feeding, respiration, reproduction and predatory impact on zooplankton community. Marine Ecology Progress Series. 2006, 314: 171-185.
Francis, R. C., & Campana, S. E. (2004). Inferring age from otolith measurements: a review and a new approach. Canadian Journal of Fish, 61: 1269-1284.
Gulland, J. A. (1969). Manual of methods for fish stock assessment. Part 1. Fish population analysis. FAO manuals in fisheries science, No. 4, 123p.
Gulland, J. A. (1983). Fish stock assessment. A manual of basic methods. Chichester John Wiley. FAO. Wiley Series on Food and Agriculture, Vol. 1: 223p.
Holt, S. J. (1965). A note on the relationship between mortality rate and the duration of life in an exploited fish population. Icnaf Research Bulletin, 2: 73-75.
Ivanov, P. I. (2000). Biological resources of the Caspian Sea. KaspNirkh, Astrakhan, 130 p.
Ivanov P. I., Kamakim A. M., Ushivtzev V. B., Shiganova T. A., Zhukova O., Aladin N., Wilson S. I., Harbinson, G. R., Dumont, H. J. (2000). Invasion of the Caspian Sea by the comb jellyfish Mnemiopsis leidyi (Ctenophora). Journal of biological invasion, 2: 255-258.
Jennings, S., Kaiser, M. J., Reynolds, D. (2001). Marine fish ecology. Black well Science Ltd., 417p.
Karimzadeh, G., Gabrielyan, B., & Fazli, H. (2010). Population dynamics and biological characteristics of kilka species (Pisces: Clupeidae) in the southeastern coast of the Caspian Sea. Iranian Scientific Fisheries Journal, 9: 422-433.
Kideys, A. E., & Moghim, M. (2003). Distribution of the alien ctenophore Mnemiopsis leidyi in the Caspian Sea in the August 2001. Marine Biology, 142: 163-171.
Mamedov, E. V. (2006). The biology and abundance of kilka (Clupeonella spp.) along the coast of Azerbaijan, Caspian Sea. ICES Journal of Marine Science, 63: 1665-1673.
Morey, G., Moranta, J., Massuti, E., Grau, A., Linde, M., Riera, F., & Morales-Nin, B. (2003). Weight-length relationships of littoral to lower slope fishes from the Western Mediterranean. Fisheries Research, 62: 89-96.
Paritskii, Y. A., KoloSyuk, G. G., & Mikhin, S. P. (2001). On the results of the Caspian kilka stocks decrease in 2000-2001. The international conference: The question of the investigation and rational use of marine natural resources. CaspNIRKH, Astrakhan, Russia, pp. 171-174.
Pauly, D. (1999). On interrelationships between natural mortality, growth parameters and mean environment temperature in 175 fish stock. J. Cons. CIEM, 39: 175-192.
Pauly, D., Soriano-Bartz, M., Moreau, J., & Jarre, A. (1992). A new model accounting for seasonal cessation of growth in fishes. Australian Journal of Marine & Fresh Water Research, 43: 1151-1156.
Pourgholam, R., Sedov, V., Yermalchev, V., Besharat, K., & Fazli, H. (1996). Stock assessment of kilka fishes by hydro acoustic method, 1994-1995. Final report, Mazandaran Fisheries Research Center, 125 p.
Prakarn, S. (2002). Building Awareness in Aspects of Fishery Statistics, Stock Assessment and Management. Proceedings of the FAO/SEAFDEC Regional Training Workshop on the Use of Statistics and Other Information for Stock Assessment. RAP PUBLICATION, 108 p.
Prikhodko, B. I. (1981). Ecological features of the Caspian kilka (genus Clupeonella). Scripta Publishing Co, pp 27-35.
Ricker, W. E. (1975). Computation and interpretation of biological statistics of fish populations. Bulletin of Fisheries Research Board of Canada, 191: 1-382.
Roohi A., Yasin Z., Kideys A., Hwai A. T. S., Ganjian Khanari A. & Develi E. (2008) Impact of a new invasive ctenophore (Mnemiopsis leidyi) on the zooplankton community of the Southern Caspian Sea. Marine Ecology, 29: 421-434.
Salmanov, M. A. (1999). Ecology and biological reproduction of the Caspian Sea. Edited by: U. I. Sorokin. Baku, 397p.
Sparre, D., & Venema, S. C. (1989). Introduction to tropical fish stock assessment. Part 1 manual. Food and agriculture organization of the united nations, Rome, Italy, 333p.
Svetovidov, A. N. (1963). Fauna of U.S.S.R fishes, Clupeidae, IPST, 2: 191-21.
Zhang, C. I., & Lee, J. B. (2001). Stock assessment and management implications of horse mackerel (Trachurus japonicus) in Korean waters, based on the relationships between recruitment and the ocean environment. Progress in Oceanography, 49: 513-537.