The freshwater butter catfish Ompok bimaculatus (Bloch, 1794), locally known as ‘Pabdha’, is an indigenous catfish belonging to the family Siluridae and order Siluriformes. It is found in India, Pakistan, Bangladesh, Myanmar, Sri Lanka and Afghanistan (Talwar & Jhingaran, 1991). However, due to reduced abundance of fish in Indian waters, it has been listed as endangered (EN) by Lakra et al. (2010). The causes behind this could be due to several factors including habitat destruction and overfishing during the breeding season (Sarkar et al., 2013; 2014), wide use of pesticides in agriculture and gradual siltation in riverine habitat.
Information available on the reproductive biology of the tropical fish O. bimaculatus from Indian waters, particularly from Ganges basin is scanty. Therefore, a thorough knowledge on reproductive biology of the butter catfish is essential for better understanding on biology as well as for evaluating the commercial potentials, life history and cultural practices (Mollet et al., 2000). Information on size at maturity is crucial to captive propagation and spawning biology (Walker, 2004; Fontoura et al., 2009). The reproductive potential of a population is one of the basic exigencies to designate the individuals of the population in respect to their gonadal conditions (Jhingran & Verma, 1972). Although, some information is available on the distribution, abundance and captive breeding of O. bimaculatus (Rao & Karamchandani, 1986; Joshi et al., 2009; Atkore et al., 2011; Mishra et al., 2013), the information on reproductive potential of different wild population of this species and intra-specific variations in reproductive patterns is not available. The determination of spawning season and strategy of reproduction within the season and the life-cycle of the fish are prerequisites in assessing the reproductive potential of a population (Jhingran & Verma, 1972) and each population can develop phenotypic and genotypic differences over the time in these parameters due to reproductive isolation (Waldman et al., 1988). In view of the above, the present study was undertaken to understand the comparative pattern of reproductive potential using sex ratio, maturity stages, body size at first gonadal maturity, spawning season, gonado-somatic index, ova diameter and fecundity in six different populations of O. bimaculatus from the Ganges basin.
MATERIALS AND METHODS
Study area and sample collection: The present study is based on the sampling and collection of 1,223 mature specimens of Ompok bimaculatus (498 males and 725 females of similar size range), made during the period January, 2011 – December, 2013 from six different tributaries of the Ganges river basin (Fig. 1 and Table 1). Specimens were collected with gill, cast, drag and fry collecting nets. Specimens were also procured from nearby landing centers of the rivers, when not available during experimental fishing. For each individual, total length (TL, in mm) as well as standard length (SL, in mm) was measured to the nearest of 0.01 cm using digital slide calipers and total bodyweight (TW, g) was taken on a digital balance (ACCULAB, Sartoriusmodel no. VIC-412,) with 0.01 g accuracy. Whole gonads were removed from mature female specimens and weighed before being preserved in 10% formalin solution.
Sexual maturity and spawning periodicity: For studying maturity and determining spawning periodicity, different maturity stages of the female O. bimaculatus were classified based on colour, shape, size of the ovary, and the space it occupied in the body cavity. The details of the criterion used and the stages of maturity and spawning in both the sexes are presented in Table 2. The maturity stages of the ovary were determined as per Rao and Karamchandani (1986), with minor modifications. The specimens were also sacrificed to check the gonadal development and different maturation stage of eggs during different months. Total weight and length of ovaries as well as testes were recorded. In addition, ova diameter and yolk deposition in the ova were also studied for recognizing the different maturity stages for female. The male specimens were sacrificed to dissect out the testes for examination.
Total body size at first gonadal maturity (L50%) and gonadosomatic index (GSI): The percentage of occurrence of various stages of maturity in different months was studied for 24 months. The spawning period was ascertained from occurrence of ripe specimens in the collected samples. Average size at first maturity (L50%) was defined as the size (the TL) at which 50% of individuals in the population reached sexual maturity during the reproductive period. The L50% was determined by modeling the proportion of mature individual according to their length class for different populations. The L50% was estimated using the logistic function expressed by the standard formula:
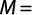

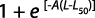

where M is the percentage of mature females by length class, L is the central value of the length class and A is the constant of the model. The suitable weight group was decided for plotting. The data collected for 24 months were pooled and the size at first maturity for both sexes was determined from the length measurements of the specimens and observation of maturity stages.
The gonadosomatic index was calculated on monthly basis using standard formula:
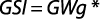

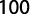
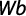
where GWg is gonad weight and Wb is total body weight.
Sex ratio: In this study, the data was pooled and the ratios of male to female were worked out month-, river- and length-wise to study the distribution of the sexes according to the season and size. To know homogeneity of the distribution of sex, Chi-square (c2) test was applied (Snedecor & Cochran, 1967).
Fecundity and egg dimension: The gravimetric method was used for studying fecundity, which was based on the relationship between ovary weight and oocyte density in the ovary (Hunter & Goldberg 1980; Murua et al., 2003). Fecundity was estimated by counting the number of mature ova from a known weight of mature/ ripe ovary. Mature female ovaries were taken for estimation of the fecundity. The sub-samples were spread evenly on a counting slide with a few drops of water and the numbers of mature ova were counted and average number of three portions was used to determine fecundity using following formula:


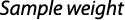
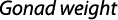
where F is the fecundity and N is the number of eggs in the sample.
To establish a relation of fecundity ‘F’ with total length ‘TL’ and total body weight ‘TW’, and ovary weight ‘OW’ following formulas (Bagenal, 1978) were used: F = aLb; F = aWb and F = a OWb; where: a and b are constants, L= total length in mm, W = total bodyweight in g and OW = ovary weight in g. The least square method was used to determine the correlation coefficient between fecundity, TL and TW.
A small portion of the ovary was taken and the diameter of the intra-ovarian eggs was measured to the nearest of 0.01 mm using Nikon SMZ 1500 stereomicroscope. The maximum oocyte diameter was obtained by taking average measurements of at least 20 of the largest oocytes, which allowed in determining the minimum and maximum diameter of the oocyte as well as distribution of oocyte size. The mean weight of an oocyte was calculated by weighing 100 oocytes using a mini scales with 100th g resolution. Only those oocytes were used which were belonging to the largest size mode in the gonads.
Statistical analysis: One-way analysis of variance (ANOVA) followed by multiple comparison Tukey test were performed on data of oocyte diameter with normal distribution and uniform variance to test differences between locations. When the data was not normally distributed and did not have uniform variance as for the fecundity and oocyte weight, a Kruskal–Wallis nonparametric ANOVA was performed to reveal significant differences in means between locations. The multiple linear regression method was applied to testify the correlation between environmental and reproductive parameters. Chi-square (c2) test was used for sex ratio. A p-value of 0.05 was used as level of significance. These tests were performed using PAST 2.17c (Hammer, 2001) and ORIGIN software packages.
RESULTS
Spawning season: In immature fishes, the testis appears delicate with very small lobules. As maturity advances, they become thick and lobulated and appear milky white in colour and oozes milky milt on slight pressure. The ovaries in female were bi-lobed and asymmetrical; immature ovaries appeared creamy yellow in colour which became slightly brownish, distended and vascularized as they became ripe. The mature eggs were glistening yellow, glossy, heavily yolked and spherical. Maturity stages were divided in to immature, maturing, mature, fully ripe and spent. Different maturity stages of fish are summarized in Table 2.
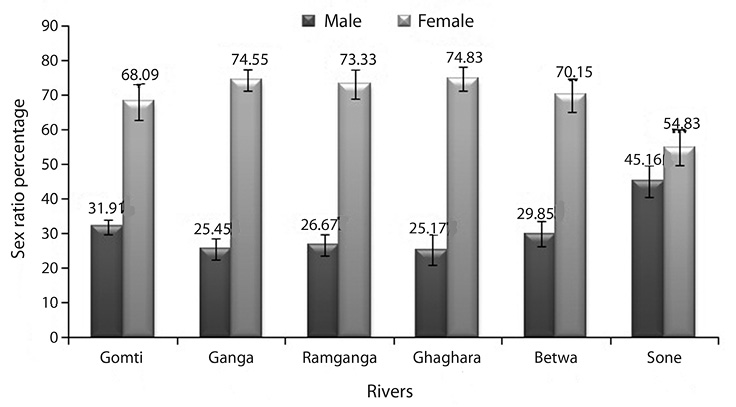
Sex ratio: River-wise data on sex ratio of 1 223 individuals of O. bimaculatus were recorded. The females were relatively higher in total body weight and total length than males. In this comparative assessment of fish, more numbers of males were found in river Sone and females in river Ghaghara (Fig. 2). The pooled data from all rivers showed that the maximum male and female sex ratio and chi square value was recorded from river Gomti and minimum in river Sone (Table 3). The chi-square test indicated no significant (p>0.05) difference in the sex ratio among the populations studied.
Sexual maturity: The distribution of female mature samples (stage IV) during spawning season (June-July) from six different populations indicated different pattern in both the years (2011-2013) and uniform distribution of stage IV was observed, except river Gomti during 2013. Out of the six populations, four populations attained above 80% maturity, except river Ganga in which it was restricted within 40% in female. However, in case of river Gomti, the status of maturity of both male and female was surprisingly different in two different years. Although, the maturity of the female was ranged from 12.83- 83.0% (mean 52.70 ± 10.33) during 2012, but very less maturity (>10%, mean 3.22 ± 1.26) was recorded during 2013 indicating possibility of breeding and recruitment failure. Most of the males attained maturity during June-July (i.e. the breeding season of fish in the Ganges basin) in both the years from six undertaken populations, showed uniform pattern.
Body sizes at first gonadic maturity (L50%): The overall size at first gonadal maturity in males was smaller as compared to the females. In females, minimum L50% was observed from the river Ghaghara and maximum from river Ramganga. In males, minimum L50% was recorded from river Ghaghara and maximum from river Sone. Based on the results obtained from pooled analysis of six female populations, the reproductive strategy may be categorized into two types: two populations attained L50% with mean TL ranged from 228 to 232 mm and four populations attained L50% with mean TL range from 242 to 262 mm. In male, out of six populations, two populations attained L50% with TL ranged from 198 to 216 mm, whereas four populations attained L50% at 227-247 mm TL (Table 5).
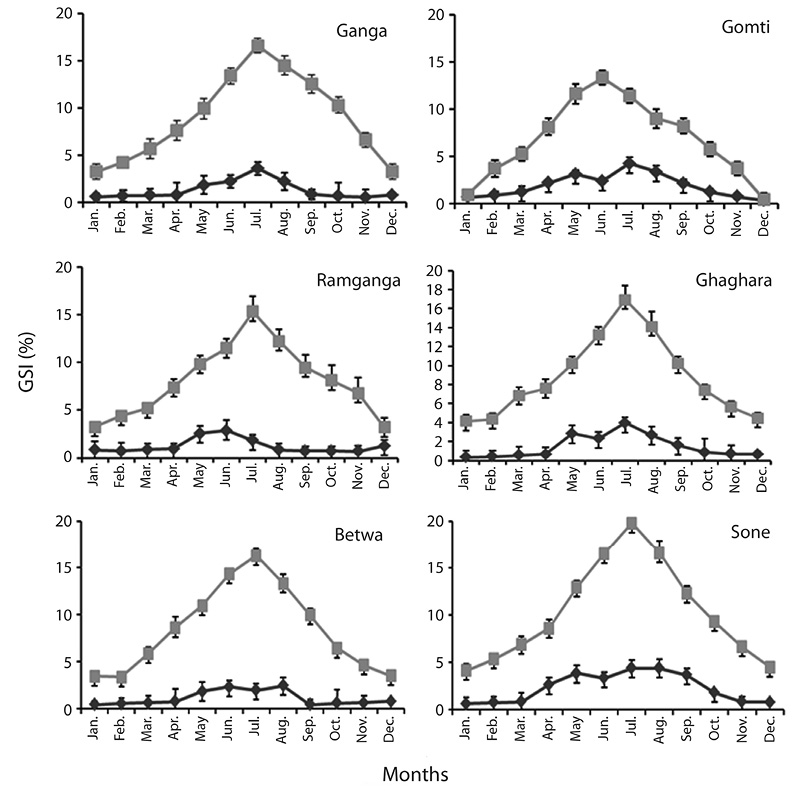
Gonadosomatic index (GSI):The month and river-wise distribution of GSI of O. bimaculatus is depicted in Figure 3. In our study, the female GSI of six population showed uniform pattern with higher value during June-August, whereas in case of male higher value was showed in four population during (June - August), while in two population this was recorded during May (Fig. 3). It is evident from the results that the GSI values increased slowly from January to March and then sharply increased in June corresponding with the spawning period. Out of the six populations, maximum GSI in males was recorded from river Sone followed by Gomti, Ghaghara, Ganga, Ramganga and Betwa, respectively. The analysis of variance (ANOVA) indicated non-significant difference in mean GSI of male and female among 6 population (p<0.08 in male, p< 0.55 in female).
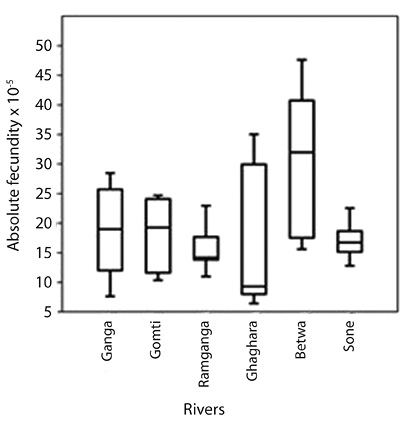
Fecundity: About 104 mature female ovaries were taken for estimation of the fecundity. About 100 mg of sub-sample was taken from three segments (anterior, middle and posterior) of each ovary with accuracy of 0.05g. In the present study, the mean absolute fecundity of fish ranged from 4,260 to 18,382 for the individuals having TL from 189.9 to 267.5 mm (Table 4 and Fig. 4). Two distinct types of reproductive patterns can be demonstrated from the six river populations: i.e. (i) high fecundity and (ii) low fecundity. Higher absolute fecundity was recorded in 5 rivers, whereas the lower absolute fecundity was recorded only in the main channel of river Ganga. Specimens sampled from river Betwa had significantly higher absolute fecundity than the other five river populations.
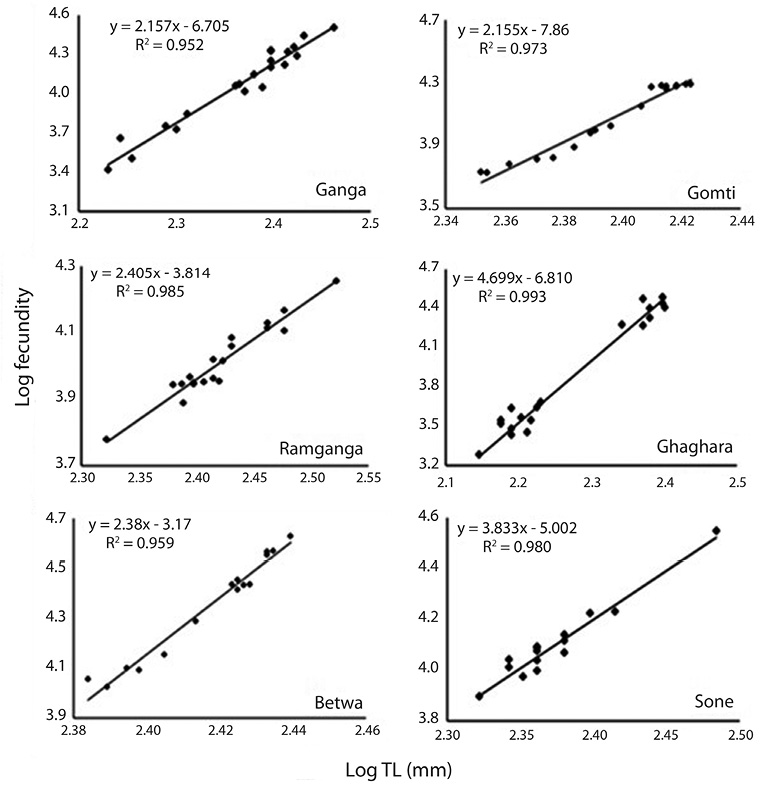
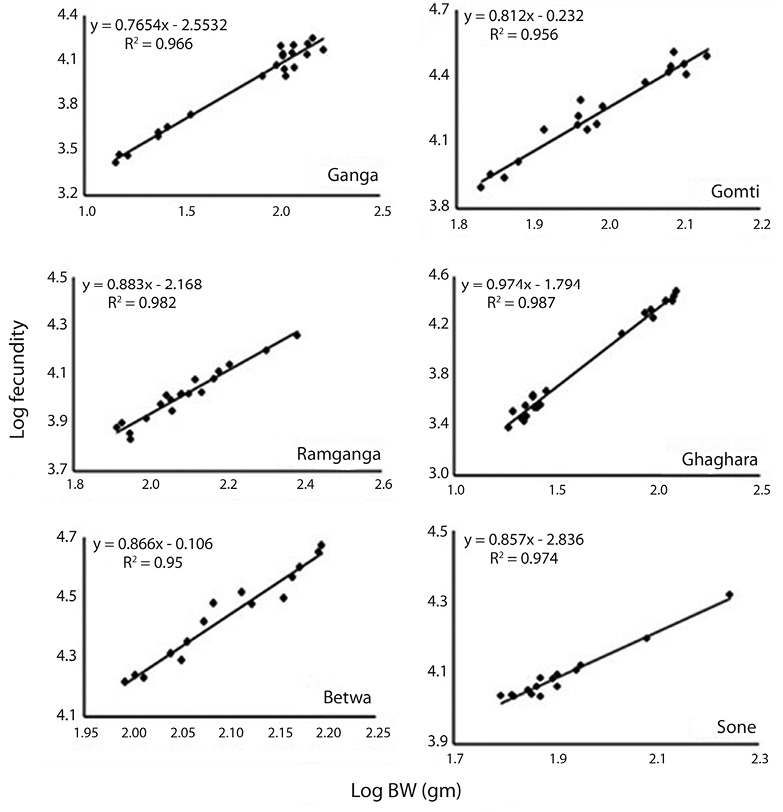
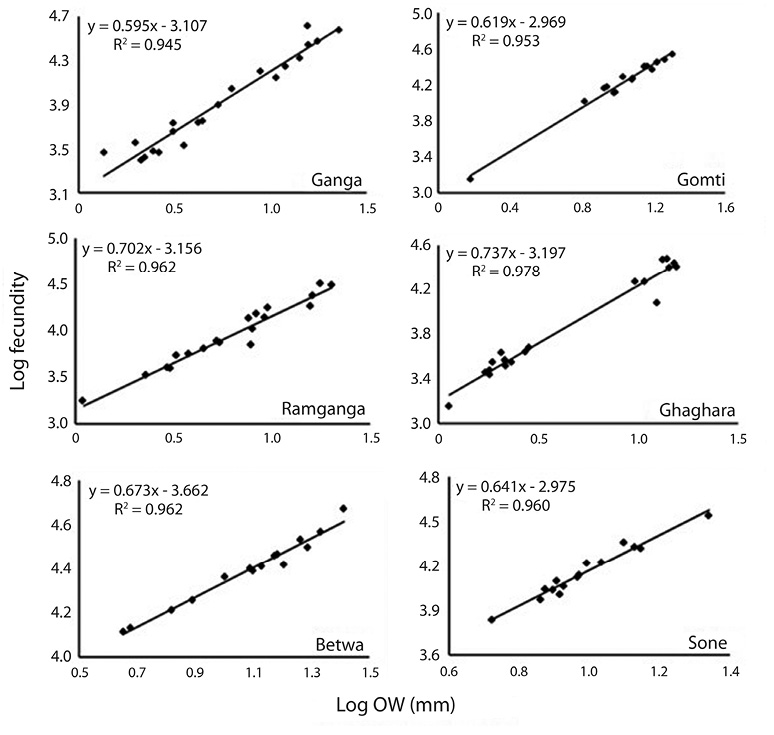
Correlation between fecundity, total length, total bodyweight and ovary weight: Table 4 showed the relationships between fecundity and total TL as well as between total TW and OW of the species. There was significant (p<0.05) and positive correlation between absolute fecundity and TL, TW and OW in all the populations. The correlation between fecundity and TL was significantly (p<0.05) stronger in river Ghaghara followed by Sone, Ramganga, Gomti, Betwa and Ganga, whereas the correlation between fecundity and TW was significantly stronger in rivers Ramganga followed by Betwa, Ganga, Gomti, Ghaghara and Sone. Rivers Ghaghara, Gomti and Sone showed significantly stronger correlations between fecundity and OW followed by rivers Ganga, Betwa, Ramganga (Figs. 5-7). Overall, it was found that the exponential value for TL–fecundity was more than that of TW–fecundity. Value for OW–fecundity was slightly lower than that of TW–fecundity.
Condition factor: The average condition factor (K) for two years was significantly (p<0.05) different during the spawning season among the rivers (Table 5). The highest K was recorded in O. bimaculatus collected from rivers Gomti, Betwa and Ghaghara. Fish caught in rivers Ganga, Ramganga and Sone had the lowest K.
Egg dimension: In ripe and gravid females, the yolked egg became rapidly differentiated and comprised around 98% of the total ova. The oocyte distribution during the fish breeding season (June -August) was almost uniform within the ovary and the ova size was observed to be maximum in size during June-July. There was no significant difference in average oocyte diameters (Table 5).
DISCUSSION
In the present study, the predominant occurrence of mature and ripe stages of female ovaries of O. bimaculatus from six different rivers during April to August indicated that O. bimaculatus has a prolonged spawning season, with individuals showing river wise variation in spawning months with a peak in June. These results also corroborate with other previous studies for O. bimaculatus in other river as reported by Mishra et al. (2013). Khumar and Siddiqui (1991) reported overall marginal dominance of males over females. The variation in sex ratio might be due to effects of different factors such as differential mortality, growth rate, longevity, sex reversal, seasons, fishing grounds and fishing methods (Pathak & Jhingran, 1977; Deepak, 2005). This deviation may also be due to patterns of migration or behavioral differences or the partial segregation of mature forms through habitat preference between males and females (Collignon, 1960; Polonsky & Tormosova, 1969; Reynolds, 1974).
The size at sexual maturity was also varied in males and females O. bimaculatus across different rivers of the Ganges basin. It was observed that male specimens attained maturity earlier than female individuals which may be attributed to the faster growth of male than females due to favorable ecological conditions and food availability (Gupta et al., 2014). The reason for this varied pattern might be due to the large varieties of food items in the reservoir which is an advantage for gonadal material production to meet the egg or milt production round the year. It was observed that female fish attained maturity earlier than male in all the river population which is similar in other Ompok species (Mishra et al., 2013; Gupta et al., 2014). The considerable variation in L50% among the different river populations can be attributed to several environmental factors, food supply and parasitism (Le Cren, 1951) and hormonal functions (Kume et al., 2009) which have great influence on the health of the fish.
In this study, percentage of gonadal maturity (stage IV) of fish in rivers Ganga (during 2012 and 2013) and Gomti (during 2013) was considerably less, which might be due to hydrological changes, overexploitation, habitat destruction and some unknown factors (Singh et al., 2010; Gupta et al., 2010). In our study, breeding activity of O. bimaculatus in the rivers was coterminous with the rainy season. Thus, rainfall and linked factor, like temperature, may act as a sign for spawning by the fish so that the offspring is produced as a time of better growth and survival. A correlation between rainfall and peak breeding activity has also been reported for other species (Admassu 1994, 1996, Teferi, 1997). In the recent years, the growing pollution in these two rivers made a noticeable change in its physical habitat and physicochemical characteristic that might be the probable cause of biological changes in fishes (Sarkar et al., 2012a). Several studies have reported the disruption in gonadal development, variation in maturity stages, malformation of the germ cells and/or reproductive ducts to altered gamete production of fishes in the rivers that received large volumes of sewage and industrial effluents (Jobling, 2002; Perera-Garcia et al., 2011).
Overall, in our study, higher absolute fecundity was recorded in all the 5 rivers, except river Ganga. The fecundity of this fish was low in comparison with the other Ompok species, for example the fecundity of O. pabda was about 30 000 (Gupta et al., 2014). This shows that the population of O. bimaculatus in the river is under stress. Variation in the fecundity of fish from six different rivers recorded in this study may depend on different factors such as protein and genetic variation, size, age, condition of the fish and food intake by the fish. Consequently, the dependency of fecundity on food supply is quite clear. Similar studies also evidences that this kind of variation in the number of eggs produced by an individual female is generally dependent on the factors like size, age, environmental conditions, food availability, vitamin and type of species (Dube, 1993; Bagenal, 1957; Bhuiyan et al., 2006). Among other different factors, like nutritional status, time of sampling and maturation stages have also been reported to affect the fecundity both within the species and among the fish populations (Sarkar et al., 2012b). The relationship of fecundity, which increased with the increase in the body parameters, TL, TW and OW of O. bimaculatus, showed positive correlation (r2= 0.95 to 0.99) in six populations studied. In previous studies, linear relationships between fecundity and weight were reported (Rao, 1981; Musa & Bhuiyan, 2007; Sarkar et al., 2009; Mir et al., 2013). Studies of Bindu et al. (2012); Buragohain and Goswami (2014) and Gupta et al. (2014) also reported significant relationships between length and body weight of fish as well as between ovary weight and fecundity in other catfish species (Horabagrus brachysoma, Clarias magur and O. pabda). The differences in condition factors among different rivers may also be attributed to low feeding intensity and degeneration of ovaries during winter and high feeding intensity and full development of ovaries at the maturity periods (Chatterjee et al., 1977; Sarkar et al., 1999; Fontoura, 2009).
The review of literature showed intra-specific differences among populations in life history traits, including age at first reproduction, fecundity etc., which can be related to thermal effects like winter mortality (Teriokhin & Budilova, 2008; Hautekeete et al., 2009) or to food availability (Jonsson & Sandlund, 1979; Walther et al., 2010). There also exists considerable evidence to suggest that population variation in life history traits reflect adaptation by fish to local environment (Taylor, 1991; Conover & Schultx, 1997). Moreover, some individuals within a population may mature earlier or at smaller size than others, often as a result of the existence of alternative reproductive strategies or in response to predation pressure. The latitudinal differences may also affect the life history traits in freshwater fishes (Gotelli & Pyron, 1991; Heibo et al., 2005; Munch & Salinas, 2009). Geuye et al. (2012) reported significant variation in reproductive traits (i.e. fecundity, oocyte diameter, oocyte weight, condition factor, GSI) of the fishes of coastal, marine, freshwater and estuarine ecosystems. In the Ganges basin, some workers have reported significant regional variation in several reproductive traits of fish (Sarkar et al., 2008, 2009; Mir et al., 2013); however, very less data is available on these complex processes.
In this study, the mean egg dimension of O. bimaculatus was comparatively higher in rivers Gomti, Betwa and Sone. The variation within different populations might be due to variation in water turbulence and proximity and population density of spawner (Denny & Shibata, 1989; Levitan & Petersen, 1995). Further, average increase in egg size and egg number might decrease as environmental quality declines and environmental variability increases (Smith & Fretwell, 1974; Einum & Fleming, 2004). Similar variations were also reported in captive reared population of another catfish O. pabda (Chakrabarti et al., 2009; Ezenwa et al., 1986). These variations were also probably due to differences in individual ovulation time and the stage of egg development (Ezenwa, 1981).
In conclusion, the present observations indicated that the wild populations of butter catfish O. bimaculatus showed considerable variation in the reproductive attributes. Hence, appropriate strategies need to be framed accordingly for its sustainable management and conservation in different drainages and tributaries of the Ganges basin.
ACKNOWLEDGMENTS
The authors are grateful to the Director, National Bureau of Fish Genetic Resources, Lucknow, for providing the necessary facilities and the Department of Biotechnology (DBT), New Delhi for granting the research project entitled ‘Identification and evaluation of reproductive traits and genetic structure of Ompok bimaculatus in India’. We would also like to specially thank fishermen of the different regions for helping in collection from different geographical locations.
REFERENCES
Admassu, D. (1994). Maturity, Fecundity, Brood size and sex ratio of Tilapia (Oreochromis niloticus L.) in Lake Awassa. Ethiopian Journal of Science,17, 53-96.
Admassu D. (1996). The breeding season of tilapia, Oreochromis niloticus L. in Lake Awassa (Ethiopian rift valley). Hydrobiologia, 337, 77-83.
Atkore, V. M., Sivakumar, K., & Johnsingh, A. J. T. (2011) Patterns of diversity and conservation status of freshwater fishes in the tributaries of River Ramganga in the Shiwaliks of the western himalaya. Current Science, 100, 73-736.
Bagenal, T. B. (1957) Annual variations in fish fecundity. Journal of the Marine Biological Association of UK, 36, 377-382.
Bagenal, T. (1978) Methods for assessment of fish production in freshwaters. Oxford: Blackwell.
Bhuiyan, A. S., Afroz, S., & Zaman, T. (2006) Food and feeding habit of juvenile and adult snakehead, Channa punctatus (Bloch.). Journal of Life and Science, 1, 53-54.
Bindu, L., Padmakumar, K. G., Sreerekha, P. S., & Joseph, N. (2012) Reproductive biology of the golden catfish, Horabagrus brachysoma (Gunther, 1864), an endemic species of the western Ghats, India. Journal of Applied Ichthyology, 28, 772-777.
Buragohain, A., & Goswami, M. M. (2014) Relationship of fecundity and different body parameters of Clarius magur (Hamilton, 1822) in captive condition in the agro climatic condition of Assam, India. IOSR JAVAS, 7, 44- 50.
Chakrabarti, P. P., Chakrabarty, N. M., & Mondal, S. C. (2009) Breeding and seed production of butter catfish, Ompok pabda (Siluridae) at Kalyani Centre of CIFA, India. Research and farming techniques, Jan.-Mar., 33-36.
Chatterjee, A., Siddiqui, A. Q., & Khan, A. A. (1977) Length-weight relationship of carp, Labeo bata (Ham). Proceedings of the National Academy of Sciences, India 86, 189-194.
Collignon. J. (1960) Contribution ia connaissance desotolihus der a frique orientale. Bulletin de l’Institut français d’études andines, 20, 55-84.
Conover, D. O., & Schultz, E. T. (1997) Natural selection and evolution of the growth rate in the early life history: what are the trades off? In: Chambers RC and Trippel (eds) Early life history and recruitments in fish populations. New York: Chapman and Hall.
Deepak, P. K. (2005) Life history traits of vulnerable Catla catla (Hamilton-Buchanan) and endangered Chitala chitala (Hamilton-Buchanan). Ph.D Thesis. Barkatullah University, Bhopal, India.
Denny, M. W., & Shibata, M. F. (1989) Consequences of surf zone turbulence for settlement and external fertilization. The American Naturalist, 134, 859-889.
Dube, K. (1993) Effect of Vitamin E on the fecundity and maturity of Heteropneustes fossilis. In: Abstract 3rd Indian Fisheries Forum. Pantnagar, India.
Einum, S., & Fleming, I. A. (2004) Environmental unpredictability and offspring size: conservative versus diversified bet-hedging. Evolutionary Ecology Research, 6, 443-455.
Ezenwa, B. (1981) A study of the reproductive biology of the catfish, Chrysichthys nigro digitatus (Lacépède) in Nigeria. (Ph.D. thesis) University of Lagos, Nigeria. 178.
Ezenwa, B., Ikusemiju. L., & Olaniyan, C. I. O. (1986) Comparative studies of the catfish, Chrysichthys nigro digitatus (Lacépède) in three isolated geographical areas in Nigeria for breeding purposes. In: Huisman EA (eds) aquaculture research in the Africa region. Wageningen, Netherlands.
Fontoura, N. F., Braun, A. S., & Milani, P. C. C. (2009) Estimating size at first maturity (L50%) from gonadosomatic index (GSI) data. Neotropical Ichthyology, 7, 217-222.
Gotelli, N. J., & Pyron, M. (1991) Life history variation in North American freshwater minnows: effect of latitude and phylogeny. OIKOS, 62, 30-40.
Gueye, M., Tine, M., Kantoussan, J., Ndiaye, P., Thiaw, O. T., & Albaret, J. J. (2012) Comparative analysis of reproductive traits in black chinned tilapia females from various coastal marine, estuarine and freshwater ecosystem PLoS One, 7, 1.
Gupta, B. K., Lakra, W. S., & Sarkar, U. K. (2010) Biodiversity, ecohydrology, threat status and conservation priority of the freshwater fishes of river Gomti, a tributary of river Ganga (India), Environmen Falist,30, 3-17.
Gupta, B. K., Sarkar, U. K., & Bhradwaj, S. K. (2014) Reproductive biology of Indian Silurid catfish Ompok pabda in river Gomti. Journal of Environmental Biology, 35, 345-351.
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001) PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontología Electrónica, 4, 9.
Hautekeete, N. C., Dijk, V. H., Piquota, Y., & Teriokhin, A. (2009) Evolutionary optimization of life-history traits in the sea beet beta vulgaris subsp. maritima: comparing model to data. Acta Oecol, 35, 104-116.
Heibo, E., Magnhagen, C., & Vollestad, L. A. (2005) Latitudinal variation in life-history traits in Eurasian perch. Ecology, 86, 3377-3386.
Hunter, J. R., & Goldberg, S. R. (1980) Spawning incidence and batch fecundity in northern anchovy, Engraulis mordax. Fish Bulletin, 77, 641-652.
Jhingran, V. G., & Verma, D. N. (1972) Sexual matutity and spawing of Gadusia chapra (Hamilton) in Ganga river system. Proceedings of the National Academy of Sciences, 42, 207-224.
Jobling, M. (2002) Environmental factors and rates of development and growth. In: Hart PJB and Reynolds JD (eds) The Handbook of fish biology and fisheries. Oxford: Blackwell.
Jonsson, B., & Sandlund, O. T. (1979) Environmental factors and life histories of isolated river stocks of brown trout (Salmo trutta fario) in Sore Osa river system, Norway. Environ. Biol. Fish, 4, 43-54.
Joshi, K. D., Biswas, B. K., Lal, S., & Vass, K. K. (2009) Piscine diversity in the river Betwa. J. Inl. Fish Soc. India, 41, 61-64.
Khumar, F., Siddiqui, M. S. (1991) Reproduction biology of teleostean fish Labeo calbasu (Ham.) from tropical lentic and lotic freshwater ecosystems. Environmental Ecology, 9, 449-455.
Kume, G., Furumitsu, K., Tanaka, S., & Yamaguchi, A. (2009) Reproductive biology of the guitarfish Rhinobatos hynnicephalus (Batoidea: Rhinobatidae) in Ariake Bay, Japan. Environ Biol Fish, 85, 289-298.
Lakra, W. S., & Sarkar, U. K. (2010) NBFGR–marching ahead in cataloguing and conserving fish genetic resources of India. Fish Chimes, 30, 102-107.
Le Cren, E. D. (1951) The length–weight relationship and seasonal cycle in gonad weight and condition in the perch (Percaflu viatilis). Journal of Animal Ecology, 20, 201-219.
Levitan. D. R., & Petersen, C. (1995) Sperm limitation in the sea. Trends Ecology Evolution, 10, 228-231.
Mir, J. I., Sarkar, U. K., Dwivedi, A. K., Gusain, O. P., & Jena, J. K. (2013) Comparative pattern of reproductive traits in Labeo rohita (Hamilton 1822) from six tropical rivers of Ganges Basin: a new insight. The Proceedings of the National Academy of Sciences, India, Section B: Biological Sciences, 84, 91-103.
Mishra, S. K., Sarkar, U. K., Trivedi, S. P., Mir, J. I., & Pal, A. (2013) Biological parameter of a silurid catfish Ompok bimaculatus from river Ghaghara, India. Journal Environmental Biology, 34, 1013-1017.
Mollet, H. F., Cliff, G., Pratt, H. L. Jr., & Stevens, J. D. (2000) Reproductive biology of the female short fin mako, Isurusoxy rinchus Rafinesque, 1810, with comments on the embryonic development of Lamnoids. Fish Bulletin, 98, 299-318.
Munch, S. B., & Salians, S. (2009) Latitudinal variation in lifespan within species is explained by the metabolic theory of ecology. Proceedings National Academy of Science, 106, 13860-13864.
Musa, A. S. M., & Bhuiyan, A. S. (2007) Fecundity on Mystus bleekeri (Day,1877) from the River Padma near Rajshahi City. Turkish Journal of Fisheries and Aquatic Sicences, 7, 161-162.
Murua, H., Kraus, G, Saborido-Rey, F., Witthames, P. R., Thorsen, A., & Jonquera, S. (2003) Procedures to estimate fecundity of marine fish species in relation to their reproductive strategy. Journal of Northwest Atlantic Fishery Science, 33, 35-54.
Pathak, S. C., & Jhingran, A. G. (1977) Maturity and fecundity of Labeo calbasu (Ham.) of Loni reservoir, Madhya Pradesh. Ibid, 9, 72-83.
Perera-Garcia, M. A., Mendoza-Carranza, M., Contreras-Sanchez, W. M., Huetra-Ortiz, M., & Perez-Sanchez, E. (2011) Reproductive biology of common snook Centropomus undecimalis (Perciformes: centropomidae) in two tropical habitats. Revista Biología Tropical, 59, 669-681.
Polonsky, A. S., & Tormosova, I. D. (1969) The Spawning of the Jack mackerel of the north east Atlantic and distribution of its eggs and larvae Trudy atlant nau chno issued. Inst Morsky Ryb Khoz Oceanography, 23, 27-48.
Rao, K. V. S. (1981) Food and feeding of lizard fishes Saurida spp. From North western part of Bangal. Journal fish, 28, 47-64.
Rao, B. J., & Karamchandani, S. J. (1986) On the spawning biology of Ompok bimaculatus (Bloch) from Kulgarhi reservoir of Madhya Pradesh. J. Inl. Fish. Soc. India, 18, 40-47.
Reynolds, J. D. (1974) Biology of small pelagic fishes in the new Volta Lake in Ghana. Part III: sex and reproduction. Hydrobiologia, 45, 489-508.
Sarkar, S. K., Medda, C., Ganguly, S., & Basu, T. K. (1999) Length-weight relationship and relative condition of bundh and hatchery-bred Labeo rohita (Ham.) during the early period of development. Asi. Fish Science, 12, 289-296.
Sarkar, U. K., Deepak, P. K., & Lakra, W.S. (2009) Stock identification of endangered clown knife fish Chitala chitala (Hamilton-Buchanan, 1822) from Indian rivers inferred by morphological attribute. E. J. Ichthyol., 2, 59-75.
Sarkar, U. K., Negi, R. S., Deepak, P. K., Lakra, W. S., & Paul, S. K. (2008) Biological parameters of the endangered fish Chitala chitala (Osteoglossiformes: Notopteridae) from some Indian rivers. Fish Res., 90,170-177.
Sarkar, U. K., Pathak, A. K., Sinha, R. K., Sivakumar, K., Pandian, A. K., Pandey, A., Dubey, V. K., & Lakra, W.S. (2012a) Freshwater fish biodiversity in the River Ganga (India): changing pattern, threats and conservation perspectives. Rev Fish Biol Fisher, 22, 251-272.
Sarkar, U. K., Kumar, R. S., Dubey, V. K., Pandey, A., & Lakra, W. S. (2012b) Population structure and reproductive biology of a freshwater fish, Labeo boggut (Sykes, 1839), from two perennial rivers of Yamuna basin. J Appl Ichthyol, 28, 107-115.
Smith, C. C., & Fretwell, S. D. (1974) The optimal balance between size and number of offspring. Am Nat, 108, 499-506.
Snedecor, G. W., & Cochran, W. G. (1967) Statistical Methods.Oxford and IBH publishing Co. Pvt. Ltd, New Delhi, 593 pp.
Talwar, P. K., & Jhingran, A. G. (1991) Inland Fisheries of India and adjacent countries. Oxford and IBH Publishing Co. Pvt. Ltd, New Delhi, pp 541.
Taylor, E. B. (1991) A review of local adaptation in salmonidae, with particular reference to pacific and Atlantic salmon. Aquaculture, 98,185-208.
Teferi, Y. (1997) The condition factor, feeding and reproductive biology of Oreochromis niloticus Linn. (Pisces: Cichlidae) in Lake Chamo, Ethiopia. M.Sc. thesis. School of Graduate Studies, Addis Ababa University, Addis Ababa, 79 pp.
Teriokhin, A. T., & Budilova, E. V. (2008) The impact of different density stresses on the dynamics of two competitive populations. Ecol. Mod., 212, 5-9.
Waldman, J. R., Grossfield, J., & Wirgin, I. (1988) Review of stock discrimination techniques for striped bass, North American. North Am. J. Fish Manage, 8, 410-425.
Walker, T. I. (2004) Elasmobranch Fisheries Management Techniques. In: Musick J. A., Bonfil R. (Eds.) Elasmobranch fisheries management techniques. APEC, Singapore, pp 285-323.
Walther, B. D., Elsdon, T. S., & Gillanders, B. M. (2010) Interactive effects of food quality, temperature and rearing time on condition of juvenile black bream Acanthopagrus butcheri. Journal Fish Biology, 76, 2455-2468.