Diversity, intensity and prevalence of parasites of Cichlids in polluted
and unpolluted sections of Eleyele Dam, Ibadan, Nigeria
I. A. Simon-Oke
Department of Biology, Federal University of Technology, Akure, Ondo State; adepejuolayemi@yahoo.com, aisimon-oke@futa.edu.ng
Received 15-viii-2016 • Corrected 30-x-2016 • Accepted 11-xii-2016
ABSTRACT: This study aimed to investigate the diversity, intensity and prevalence of parasites in Cichlids. A total of 354 specimens of cichlids was sampled in polluted and unpolluted ends of Eleyele River, Ibadan, Nigeria and examined for parasites. The total prevalence was 57.34%. Recovered parasites were Clinostomum tilapiae, Euclinostomum heterostomum, Neascus, Allocreadium ghanensis, Phagicola longa, Alloglossidium corti and the acanthocephalan; Acanthogyrus tilapiae and Acanthella. Clinostomum tilapiae had higher prevalence and abundance (42.90%) with Allocreadium corti recording least abundance (0.49%). Tilapia zilli was the most abundant (41.53%) among the fish hosts but Oreochromis niloticus harboured the highest percentage of parasites (80.00%). The fish hosts in the polluted end of the river harboured the highest percentage of parasites (71.18%) against (43.50%) parasites recovered from the unpolluted end. There was a significant difference in the parasites harboured. Heavy infection with a broad number of parasites in fish hosts could reduce performance and productivity of the species, especially in fish farming.
Key words: Cichlids, Parasites, Polluted, Unpolluted, Eleyele Dam.
Fish farming occupies a position second only to crop production in the food sector in Nigeria (Gnadadoss, 1980). Fish culture provides a large reservoir of parasitic pathogen common to both wild and cultured fish. With the growing interest in the development of fish farming and culture especially in the tropics, there is an increasing awareness of the importance of fish diseases as one of the detrimental factors in the venture. High population of fish culture favours the spread of many diseases and parasites (Anyanwu, 1983).
Parasite infections in fishes cause production and economic losses through direct fish mortality, reduction in fish growth, fecundity and stamina, increase in the susceptibility of fish to diseases and predation and the high cost of treatment (Cowx, 1992). Many phyla of the animal kingdom have representatives that are parasites of cichlids, where the helminthes are a major group of parasites involved (Awa et al., 1996). Helminthes are among the most important parasites which include nematodes, trematodes, cestodes and acanthocephalans affecting both wild and cultured fishes (Hussen et al., 2012). The commonest infection are caused by trematodes: Clinostomum sp., Euclinostomum sp and nematode Procamellanus sp. Ukoli (1992) and Okaeme (1991) also reported this observation.
The life history of digeneans which parasitize fish involves three hosts; an aquatic snail (first intermediate host), fish (second intermediate host) and a fish eating bird such as Heron (final host) (Ukoli, 1992). He also reported the susceptibility of Oreochromis niloticus, Sarotherodon galilaeus and Tilapia zilli to Clinistomum tilapiae infection. Acanthocephalan (spiny- headed worms) have a characteristic retractable proboscis at the anterior end which is armed with posterior pointing hooks. This organ of attachment anchors the worm in the intestine wall of the fish host (Egusa, 1992).The larval stages of Acanthocephalans occur within crustaceans or insects. Severe outbreaks have occurred in fish farms where large number of infected shrimps were introduced in an effort to improve the growth rate of fish (Roberts and Shepherd, 1996). Pathogenic effects arise from abrasions and lesions caused by the proboscis during attachment. When it penetrates only the intestinal epithelium, the host reaction is minimal weight loss, growth retardation and mortality of fish occur if the parasites are present in large numbers (Cowx, 1992).
Parasites disease of fish (and livestock) reduces the amount of food available to people around the globe. It is imperative therefore to investigate the relationship between the environmental factors as it affects the parasites that affect production and quality. Due to the importance of fish as one of the major source of obtaining cheap protein, studies on this aspect of biology, morphology and diseases of fish is very important. This study was therefore conducted to provide information on the endo parasites of Cichlids because of their importance in the artisanal fisheries in Nigeria.
MATERIALS AND METHODS
Study area: Eleyele Dam which picks its source from river Ona is located between latitudes 7°26’ N and longitudes 3°52” E with an altitude of 125m above sea level. Seasonal temperature occurs with the mean minimum temperature (24.5oC) occurring in August when there is dense cloud cover. The climatic condition of the study area is characterized by two distinct seasons, the wet and dry seasons respectively, the mean annual rainfall is 1262.3mm and the vegetation is typical rain forest. The inhabitants of the area speak mainly Yoruba language and are basically fishermen and farmers.
Collection, Identification and Processing of Fish samples for parasite examination: A total of 354 live Tilapia species were collected at the two ends forth nightly and samples were collected between 0700 and 1000hours at each end as recommended by Adebisi (1981).The fishes were caught by the fishermen using cast nets (mesh size, 4cm2) which was set in the evening and retrieved the following morning. The fishes were placed in an ice-chest and trans-ported to the Fishery laboratory in the Department of Zoology, University of Ibadan for identification, processing and examination for parasites. The fish samples were identified using Field Guide to Nigerian Fresh Water Fishes by Olaosebikan and Raji (1998).
The sexes of the fishes were determined by either the presence or absence of an intromittent organ on the ventral side just before the anal fin which as confirmed later by the presence of testes or ovaries during dissection. The total length and standard length measurements were taken using a calibrated dissecting board. The weight of each fish was taken using a weighing balance (metler 0.01g). The skin, scales, eyes and gut of the fishes were all removed with the aid of a dissecting tool and these organs were immediately immersed in a saline water to aid the emergence of parasites.
Excystment of Cysts: Helminths cysts were excysted by subjecting them to slight increase in temperature in a bile solution as medium (Wright, 1977).
Preservation of Parasites: The parasites were allowed to die and stretch fully in normal saline water for 30min after which they were fixed in Alcohol- Formalin-Acetic Acid (AFA) solution for 24hours and later preserved in 70% alcohol with two drops of glycerine to prevent contraction of the worms and complete evaporation.
The staining method was used for the treatment of Acanthocephala where the parasites were dehydrated using alcohol at different concentrations: 70% alcohol, 85% alcohol, 95% alcohol and 100% alcohol for a period of 10min each. After dehydration, the parasites were cleared in xylol and mounted in Canada balsam (MAFF, 1971). The slides were observed under a light microscope and the parasites identified using information provided by Yamaguti, 1963).
Statistical analysis: The relationship that exists between the parasite burden and other tested variables (length, weight and sex) were compared using correlation analysis and t-test. P-values equal to or less than 0.05 were considered significant (Steel & Torrie, 1980).
RESULTS
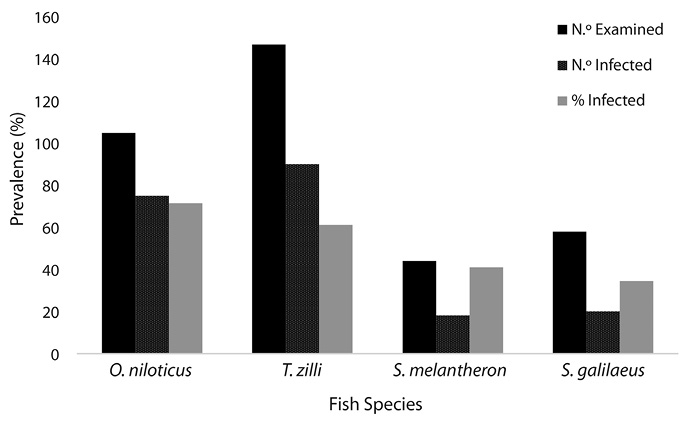
Figure 1 shows that Oreochromis niloticus had the highest number of parasite infestation followed by Tillapia zilli and the least infection was found in Sarotherodon galilaeus. Statistical analysis showed that there was no significant difference in number of infected fishes (P> 0.05).
Table 1 shows that the females were more infected with parasites than the males except in S. melanotheron where the males recorded 64.28% infection against the females 30.00%. However, there was no statistical difference (p>0.05) in infection according to their sexes.
Table 2 shows that the fish hosts in the polluted end of the river harboured more parasites than the fish hosts at the unpolluted end. There was a significant difference in the level of infection at both ends. At the both ends, O. niloticus recorded the highest number of infection (84.9%) and (57.7%) while the least were recorded in S. galilaeus (31.0%) and S. melanotheron (31.8%) respectively.
The fish hosts with sizes 21-50.9 to 111-140.9 recorded the highest percentage of infection with the least recorded in size 10-20.9 (Table 3).
According to the location of the parasites in the fish hosts, the intestine harboured most of the parasites which are mainly Trematodes while the gonads and the mouth harboured the least parasites (Table 4). Among all the parasites recovered, Clinostomum tilapiae was the most abundant with the least in Allocreadium ghanensis. There was a significant difference (p<0.05) in the abundance of the parasite species.
DISCUSSION
The study showed a high infection rate (57.34%) in all the fishes examined. This is similar to the findings of Amaechi (2015) in Ilorin, South western Nigeria. Six species of helminths, Euclinostomum heterostomum, Clinostomum tilapiae, Neascus, Allocreadium ghanensis, Phagicola longa and Alloglossidium corti and Two Acanthocephalan; Acanthogyrus tilapiae and Acanthella were the parasites recovered. These parasites have also been recovered from cichlids and other fresh water species by (Ukoli 1970; Olurin and Somorin, 2006; Ohaeri (2012) who noted that the occurrence of E. heterostomum and C. tilapiae were widespread amongst cichlids. The trematodes recovered were most abundance in the intestine which are associated with the digestive activity that normally results in the release of parasite oval/ cysts in food particles.
Hussen et al., (2012) reported that helminths are mostly found in fresh water fishes where factors such as parasite species and its biology, host and its feeding habitats, physical factors, hygiene of the water body and presence of intermediate hosts contribute to their prevalence and intensity. Clinostomum tilapiae larvae are the dominated parasites species of O. niloticus observed in this study. It was observed beneath operculum, in pharyngeal region and on gill. This result is similar to those of Aloo (2002) and Ochieng et al., (2012) who observed Clinostomum spp. below the operculum and in the pharyngeal region in Oreochromis leucostictus in Lake Naivasha. O. niloticus feeds mainly on benthic materials, including detritus, by picking up larval stages of parasites. The metacercariae of Clinostomum in the specimens of fish host suggested the presence of snails in the sites of study which are the first intermediate hosts of parasites (Clinostomum). The metacercariae of Clinostomum is known to damage the muscles of fish, making it degusting and unsalable (Coulibaly, 1995).
Acanthella and Acanthogyrus tilapiae are the Acanthocephalan parasites recovered and were restricted to the fish intestine. Differences in physical environment in the gut, availability, nature, and amount of food supply were factors that most likely limit the distribution of parasites in different sections of alimentary tract (Nkwengulila and Mwita, 2004). Hence, the preference of Acanthocephalans for intestinal region as site of attachment could be attributed to food availability in this region. Acanthocephalans do not have a gut, nutrients from the lumen of the host gut are absorbed across the body wall of the parasites. Amin et al. (2008) reported that test results suggest that A. tilapiae was better adapted to some cichlid hosts than to others. In this study, more females were infected than males (p<0,05).This could be attributed to differential feeding pattern which could be in terms of quality and quantity. It could also be attributed to differences in the degree of resistance to infection. However, this result disagrees with the findings of Olurin et al., (2012) and Biu and Nkechi, (2013).
REFERENCES
Adebisi, A. A. (1981). The physiochemical hydrology of a tropical seasonal River-upper Ogun River. Hydrobiologia, 7, 157-167.
Aloo, P. A. (2002). A comparative study of helminth parasites from the fish Tilapia zilli and Oreochromis leucostictus in Lake Nairasha and Oloiden Bay, Kenya. Journal of Helminthology 76(2), 95-104.
Amin O. M., Oosterhout C. V., Blais J., Robinson R. L. (2008). On the Ecology and Host relationships of Acanthogyrus (Acanthosentis) tilapiae (Acanthocephala: Quadrigyridae) from Cichlids in Lake Malawi. Comp. Parasitol. 75(2): 278-282.
Anyanwu, A. O. (1983). Parasitic infestations of Pseudotolithus spp of the coast of Lagos, Nigeria. J. Fish Biol. 22: 29-33.
Awa, J., Anyanwu, P., & Ezenwa, B. (1988). Incidence of Parasitic infection of pond raised Tilapia spp. And some cultivable fish species from three ecological areas of Lagos State. NIOMR Tech. Pap., (32), 24.
Biu, A. A., & Nkechi, O. P. (2013). Prevalence of Gastrointestinal Helminths of Tilapia zilli (Gervais 1848) in Maiduguri, Nigeria. Nigerian Journal of Fisheries and Aquaculture, 1 (1), 20-24.
Coulibaly N. D., Salembéré S., & Bessin R. (1995). La clinostomose larvaire des poissons Cichlidés du lac de la Kompienga au Burkina Faso: une ménace potentielle pour l’exploitation halieutique et la santé publique. Cah. Santé, 5(3), 189-193.
Cowx, I. G. (1992). Aquaculture development in Africa, training and Reference manual for Aquculture Extensionists. Food production and Rural Development Division. Common Wealth Secretariat London. 246-295.
Ebube. C. Amaechi (2015). Prevalence, Intensity and Abundance of endoparasites in Oreochromis niloticus and Tilapia zilli (Pisce: Cichlidae) from Asa Dam, Ilorin, Nigeria. Cuadernos de Investigation UNED, 7(1).
Egusa, S. (1992). Infectious diseases of Fish Ist edition. Balkema Pubis; Brookfied, U.S.A. 696.
Gnadados, A. S. (1980). Proceedings of the National Seminar on Integrated Development of Artisanal and Inshore Fisheries in Nigeria. 83 pp.
Hussen, A., Tefera, M., & Asrate, S. (2012). Gastrointestinal hel-minth parasites of Clarias gariepinus (Catfish) in Lake Hawassa Ethiopia. Scientific Journal of Animal Science 1(4), 131-136.
Maff (1971). Manual of Veterinary Parasitologia Laboratory Techniques. Ministry of Agric. Fisheries and Food, London. Technical Bulletin, 18 pp.
Nkwengulila G., Mwita C. (2004). Spatial distribution of parasites along the gut of the catfish Clarias gariepinus (Burchell, 1822) (Clariidae) from the Mwanza gulf, lake Victoria. Tanzan. J. Sci., 30(1), 63-70.
Ochieng V. O., Matolla G. K., & Khyria S. K. (2012). A study of Clinostomum affecting Oreochromis niloticus in small water bodies in EldoretKenya. Int. J. Sci. Eng. Res. 3(4), 1-6.
Ohaeri, C. C. (2012). Gut helminthes parasites and host in-fluence in Nile Tilapia, Oreochromis niloticus. Journal of Biological Science and Bioconservation, 438-43.
Olaosebikan, B. D., & Raji, A. (1998). Field Guide to Nigerian Freshwater Fishes. Federal College of Fresh Water Fisheries Technology New Bussa, Niger State, Nigeria. 106 pp.
Olurin, K. B., & Somorin, C. A. (2006). Intestinal helminthes of the fishes of Owa stream, south west Nigeria. Research Journal of Fisheries and Hydrobiology 1 (1), 6-9.
Olurin, K. B., Okafor, J., Alade, A., Asiru, R., Ademiluwa, J., Owonifari, K., & Oronaye, O. (2012). Helminth parasites of Sarotherongalilaeus and Tilapia zilli (Pisces: Cichlidae) from River Oshun, South west Nigeria. International, Journal of Aquatic Science 3 (2), 49-55.
Roberts, R. J. & Shepherd, C. J. (1986). Handbook on Trout and Salmon diseases. 2nd edition. Blackwell Sci. Publs. Ltd. London. 222 p.
Steel, R. G. D., & Torrie, J. H. (1980). Principles and Procedures of Statistics 2nd ed. New York: Mac GrawHill.
Ukoli, F. M. A. (1970). On the adhesive mechanism of Apharyngostriages simplex and Clinostomum tilapiae. Nigerian Journal of Science, 477-79.
Ukoli, F. M. A. (1992). Introduction to Parasitology in Tropical Africa. Text flow Ltd; Ibadan, Nigeria. 404 pp.
Wright, R. A. (1971). Flukes and Snails Allen and UNNIN Ltd.
Yamaguti, S. (1963). Systema Helminthum, the Acanthocephans New York: Interscience Publishers.
RESUMEN: Diversidad, intensidad y prevalencia de parásitos de los cíclidos en secciones contaminadas y no contaminadas de la Represa de Eleyele, Ibadan, Nigeria. Un total de 354 especimenes de ciclidos se muestrearon en los extremos contaminadas y no contaminadas del río Eleyele, Ibadan, Nigeria y se examinaron sus parásitos. El total de prevalencia fue del 57.34%. Los parásitos encontrados fueron Clinostomum tilapiae, Euclinostomum heterostomum, Neascus, Allocreadium ghanensis, Phagicola longa, Alloglossidium corti y Acanthogyrus tilapiae, Acanthella. Clinostomum tilapiae tuvo la mayor prevalencia y abundancia (42.90%) y Allocreadium corti con la menor abundancia (0.49%). Tilapia zilli fue el más abundante (41.53%) entre los hospederos de los peces pero Oreochromis niloticus albergó el mayor porcentaje de parásitos (80%). Los hospederos de peces en el extremo contaminado del río contienen el mayor porcentaje de parásitos (71.18%) contra (43.50%) de parásitos recuperados en el extremo no contaminado. Existe una diferencia significativa en los parásitos albergados. Una infección intensa con un amplio número de parásitos en peces hospederos podría reducir el rendimiento y la productividad de la especie, especialmente en la piscicultura.
Palabras clave: cíclidos, parásitos, contaminado, no contaminado, represa Eleyele.
Fig. 1. Infection Rate of Fish Hosts.
TABLE 1
Relationship between the Sex of Fish Hosts and Infection Rate
|
Fish spp |
No Examined |
No Infected |
% Infection |
|||
|
Male |
Female |
Male |
Female |
Male |
Female |
|
|
O. niloticus |
70 |
35 |
47 |
28 |
67.14 |
80.0 |
|
T. zilli |
83 |
64 |
50 |
40 |
60.24 |
62.5 |
|
S. melanotheron |
14 |
30 |
09 |
09 |
64.28 |
30.0 |
|
S. galilaeus |
37 |
21 |
09 |
11 |
24.32 |
52.38 |
|
Total |
204 |
150 |
115 |
88 |
56.37 |
58.67 |
X2 cal= 5.333a, x2 tab= 0.004, df= 1, P= 0.377.
TABLE 2
Prevalence of Parasites at the Polluted and Unpolluted End of the Study Area
|
Fish Host |
Polluted End |
Unpolluted End |
||||||
|
N.º Examined |
N.º Infected (%) |
Parasite Recovered |
Mean No per Infected Host |
N.º Examined |
N.º Infected (%) |
Parasite Recovered |
Mean No per Infected Host |
|
|
O. niloticus |
53 |
45(84.9) |
90 |
0.043 |
52 |
30(57.7) |
58 |
0.058 |
|
T. zilli |
73 |
61(83.6) |
84 |
0.031 |
74 |
29(39.2) |
51 |
0.058 |
|
S. melanotheron |
22 |
11(50.0) |
43 |
0.148 |
22 |
7(31.8) |
48 |
0.240 |
|
S. galilaeus |
29 |
9(31.0) |
61 |
0.198 |
29 |
11(37.9) |
23 |
0.123 |
|
Total |
177 |
126(71.18) |
278 |
|
177 |
77(43.50) |
180 |
|

TABLE 3
Size of Fish hosts (cichlids) in relation to Infection with parasites
|
Fish |
Weight (g) |
N.º Examined |
N.º Infected (%) |
% Mean per Infected Host |
% Mean Per Host |
|
O. niloticus |
10 – 20.9 |
25 |
14 (56.0) |
18.69 |
7.15 |
|
T. zilli |
|
23 |
11(47.82) |
16.36 |
9.09 |
|
S. melanotheron |
|
5 |
2(40.0) |
16.80 |
50.00 |
|
S. galilaeus |
|
8 |
2(25.0) |
17.60 |
50.00 |
|
O. niloticus |
21 – 50.9 |
38 |
29(76.31) |
40.05 |
3.45 |
|
T. zilli |
|
42 |
24(57.14) |
41.48 |
4.17 |
|
S. melanotheron |
|
7 |
3(42.86) |
46.93 |
33.33 |
|
S. galilaeus |
|
14 |
3(21.43) |
45.58 |
33.33 |
|
O. niloticus |
51 – 80.9 |
19 |
16(84.21) |
70.02 |
6.25 |
|
T. zilli |
|
28 |
17(60.71) |
74.34 |
5.88 |
|
S. melanotheron |
|
18 |
6(33.33) |
74.63 |
16.67 |
|
S. galilaeus |
|
20 |
9(45.00) |
62.75 |
11.11 |
|
O. niloticus |
81 – 110.9 |
15 |
10(66.66) |
93.20 |
10.00 |
|
T. zilli |
|
43 |
29(67.44) |
93.45 |
3.45 |
|
S. melanotheron |
|
6 |
3(50.00) |
103.6 |
33.33 |
|
S. galilaeus |
|
11 |
3(27.27) |
97.37 |
33.34 |
|
O. niloticus |
111 – 140.9 |
8 |
6(75.00) |
124.93 |
16.67 |
|
T. zilli |
|
11 |
9(81.81) |
119.11 |
11.11 |
|
S. melanotheron |
|
8 |
4(50.00) |
115.83 |
25.00 |
|
S. galilaeus |
|
5 |
3(60.00) |
156.83 |
33.33 |
TABLE 4
Distribution pattern, Location and Parasites recovered in Fish Hosts
|
Parasite |
Taxonomic Group |
Location |
N.º of Infected Fish (%) |
N.º of Parasites |
|
Clinostomum tilapiae |
Trematoda |
Operculum, gills, intestine, body cavity& gonads |
87(42.9) |
206 |
|
Acanthogyrus tilapiae |
Acanthocephala |
Intestine |
24(11.8) |
51 |
|
Euclinostomum heterostomum |
Trematoda |
Intestine |
10(4.93) |
22 |
|
Acanthella |
Acanthocephala |
Intestine |
35(17.2) |
79 |
|
Neascus |
Trematoda |
Intestine |
10(4.93) |
26 |
|
Allocreadium ghanensis |
Trematoda |
Intestine and Mouth |
11(5.42) |
21 |
|
Phagicola longa |
Trematoda |
Intestine |
25(12.3) |
50 |
|
Alloglossidium corti |
Trematoda |
Intestine |
1(0.49) |
3 |
|
Total |
|
|
203 |
458 |