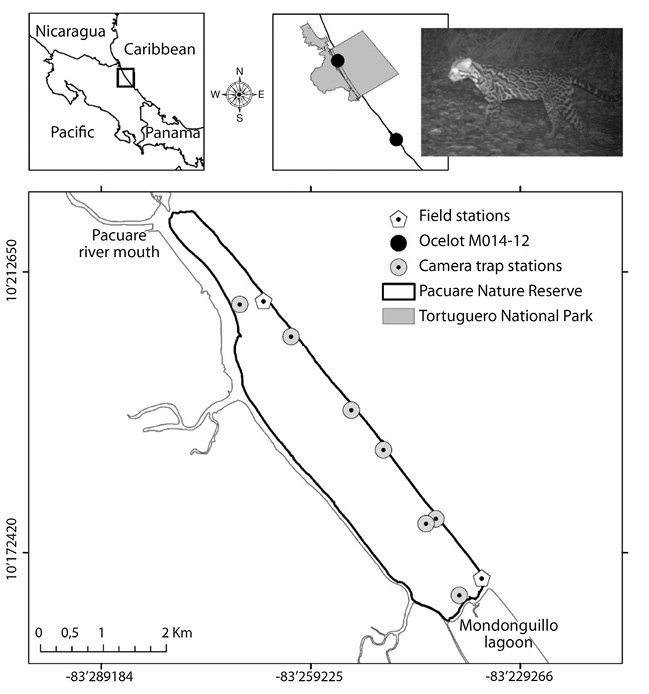
Fig. 1. Study area and camera trap distribution in Pacuare Nature Reserve, Costa Rica.
Relative abundance and activity patterns of terrestrial mammals
in Pacuare Nature Reserve, Costa Rica
Stephanny Arroyo-Arce1, Ian Thomson1, Carlos Fernández2 & Roberto Salom-Pérez3
1. Coastal Jaguar Conservation, 126-3100 Santo Domingo, Heredia, Costa Rica; sturnina@gmail.com
2. Endangered Wildlife Trust, Barrio Escalante, Avenida 7, Calles 33 y 35, San José, Costa Rica.
3. Panthera, 8-3870-1000 San José, Costa Rica.
Received 13-iX-2016 • Corrected 30-ix-2016 • Accepted 10-XX-2016
ABSTRACT: Located in Costa Rica, Pacuare Nature Reserve has a long established history of wildlife monitoring programs primarily focused on species of nesting marine turtles and the Agami herons (Agamia agami) found within the reserve. Our research represents the first assessment on the local terrestrial mammal populations. Data was collected by using seven camera trap stations distributed within the boundaries of the reserve. From April 2015 to March 2016, and after a total of 1 643 camera trap nights, we were able to identify 11 terrestrial mammalian species distributed in six orders and nine families. The most abundant species was the common opossum (Didelphis marsupialis), followed by the ocelot (Leopardus pardalis). A noticeably absent species, otherwise common throughout the area, was the Central American agouti (Dasyprocta punctata). Our results are similar to those from other protected areas in the Northeastern Caribbean coast of Costa Rica.
Key words: mammals; relative abundance; activity pattern; camera trapping; Costa Rica.
Wildlife monitoring has proven to be an essential tool for assessing the condition of ecosystems. In the Neotropics, it has been used widely to gather baseline information such as presence of threatened species (Beisiegel, 2009; Mérida & Cruz, 2015), estimate species richness and abundance (García, Delgado-Jaramillo, Machado & Aular, 2012; Arévalo, Méndez, Roberts, Alvarado & Vargas, 2015) and assess the impacts of human disturbance on a species (Navarro & Gómez, 2015). Furthermore, when applied in long-term monitoring projects it can be used to evaluate spatial and temporal trends at a local, regional and global level (Ahumada et al., 2011; Altrichter et al., 2011; Ahumada, Hurtado, & Lizcano, 2013), this information is essential to improve conservation and management strategies.
In the Pacuare Nature Reserve wildlife monitoring has focused mainly on the nesting population of marine turtles (Cuskelly, 2012; Sarmiento-Ramírez et al., 2014; Rivas, Fernández & Marco, 2015a; Rivas, Santidrián, Diéguez & Marco, 2015b). Other studies have described the reproductive phenology of the Agami heron (Agamia agami; Abella-Gutiérrez & López-Conlon, 2008), and assessed foraging competence in the white-faced capuchin (Cebus capucinus; Eadie, 2015). To date, research on terrestrial mammals has not been conducted. In this paper, we present the first camera trap study of mammalian species in Pacuare Nature Reserve. We collected baseline information on species richness of mammals, as well as information on relative abundance and activity patterns of some terrestrial species.
MATERIALS AND METHODS
Study site: Pacuare Nature Reserve is a private reserve located on the Caribbean coast of Costa Rica (10o10’00’’ N - 83o14’00’’ W; Fig. 1). Established in 1989, it covers an area of 1050 ha where the predominant ecosystem is the Lowland Rainforest. The Reserve is bordered by Pacuare river mouth in the North, and the Mondonguillo lagoon to the South (Abella-Gutiérrez & López-Conlon, 2008; Rivas et al., 2015a; Rivas et al., 2015b).
The Reserve includes 6 km of coastline that constitutes a key nesting site for the vulnerable leatherback turtle (Dermochelys coriacea; Troëng, Chacón & Dick, 2004; Chacón-Chaverri & Eckert, 2007; Wallace, Tiwari & Girondot, 2013). It also hosts a small nesting population of the endangered green turtle (Chelonia mydas; Seminoff, 2004) and the critical endangered hawksbill (Eretmochelys imbricata; Mortimer & Donnelly, 2008).
Camera trapping: Seven camera trap stations were set in the study area, between April 2015 and March 2016. All stations were located along a human-made trail running parallel between the beach and the forest, as well as in secondary trails (Fig. 1). Each station consisted of one or two digital camera trap (Bushnell Trophy Cam SD or Bushnell Trophy Cam HD) installed at a height of 60cm. The cameras were programmed to record photos (3 photos) and video (10s) at 3s intervals. Each station had a scent lure (Chanel N°5) placed 2-5m in front of the camera in an attempt to increase the time animals stood in front of the camera, and thus facilitating identification of species and individuals (e.g. coat pattern, wounds, scars, skin parasites). Stations were checked on a monthly basis to ensure the equipment was still functioning, replace batteries and memory cards, clear vegetation and refresh scent stations.
Data analysis: Relative abundance index (RAI) for every mammalian species was determined using Equation 1.
Equation 1
RAIspa=events*100 camera trap nights/sampling effort.
Where RAIspa=relative abundance index for species ‘a’; events=number of independent records per species; 100 camera trap nights=unit of standardization to compare data with other studies; sampling effort=total amount of nights that the camera trap stations were working.
Following Springer et al. (2012) only one individual per species was counted at each camera trap station within a 24h period (independent record); with the exception of gregarious species and felids. For gregarious species, when instances of more than one individual were recorded at the same time and for the same station, the number of independent records was considered equal to the number of individuals observed in the same frame (Monroy-Vilchis, Zarco-González, Rodríguez-Soto, Soria-Díaz & Urios, 2011). For felids (or other species where individuals could be identified) we considered consecutive records of the same individual, at the same station, as independent records when there was a 24h interval between detections (Monroy-Vilchis et al., 2011).
Activity patterns were estimated for species with 11 or more independent records (Maffei, Noss, Cuéllar & Rumiz, 2005; Monroy-Vilchis et al., 2011). Records were grouped in two hours intervals, and the activity patterns were classified as diurnal (8:00-18:00h), nocturnal (20:00-6:00h) and crepuscular (6:00-8:00h and 18:00-20:00h). Species that showed sporadic and random intervals of activity were classified as cathemeral (Maffei et al., 2005; Monroy-Vilchis et al., 2011).
RESULTS
After a total of 1 643 camera trap nights, we were able to identify 11 terrestrial mammalian species distributed between six orders and nine families (Table 1). The most common mammals recorded were carnivores (three families and five species) of which three species were wild cats (Panthera onca, Puma concolor and Leopardus pardalis). A total of five jaguars (P. onca) were identified, including two adult males, one female with her cub and one unsexed adult. Only one photographic record of an adult male puma (P. concolor) was recorded during the study period. Regarding the ocelot (L. pardalis) two adult individuals were identified, a male and a female. It is important to highlight that the male M014-12 (recorded in July 2015 at Pacuare Nature Reserve) was previously detected in Tortuguero National Park in November of 2012 (Coastal Jaguar Conservation, unpublished), travelling a linear distance of approximately 41km (Fig. 1).
With the exception of the common opossum (Didelphis marsupialis), the other species of opossums were not possible to identify to the species level due to the poor quality of the photos and videos, however it is suspected that they are either the gray four-eyed opossum (Philander opossum) or the brown four-eye opossum (Metachirus nudicaudatus); these records were not included in the data analysis.
According to the ‘relative abundance index’, the most abundant mammalian species were the common opossum (D. marsupialis) and the ocelot (L. pardalis), while the puma (P. concolor) and the Northern tamandua (Tamandua mexicana) were the least abundant species recorded in the area, respectively (Table 1).
Activity patterns were estimated for five species of mammals (Fig. 2). Our results suggest that the jaguar (P. onca), the ocelot (L. pardalis), the common opossum (D. marsupialis) and the spotted paca (Cuniculus paca) are mainly nocturnal, while the red brocket deer (Mazama temama) was classified as diurnal and nocturnal.
Two arboreal mammals were also recorded including the white-faced capuchin (Cebus capucinus) and the Geoffroy’s spider monkey (Ateles geoffroyi). Although Baird’s tapir (Tapirus bairdii) was not recorded on the camera traps, its tracks were observed along the trails. In addition to mammals, we also recorded six species of birds (Tigrisoma mexicanum, Agami agami, Mesembrinibis cayennensis, Crax rubra, Aramides cajaneus, Buteogallus anthracinus) and two species of reptiles (Iguana iguana and an unidentified tortoise).
DISCUSSION
Terrestrial mammal assemblage documented in the reserve were similar to those reported in other protected areas located along the Northeastern Caribbean coast of Costa Rica, including Tortuguero National Park (Arroyo-Arce, Guilder & Salom-Pérez, 2014; Coastal Jaguar Conservation, unpublished) and Barra del Colorado Wildlife Refuge (Arroyo-Arce, Thomson & Salom-Pérez, 2016). The major discrepancy between these areas was the absence of the Central American agouti in the reserve, despite of been one of the most abundant species in the adjacent areas (Coastal Jaguar Conservation, 2016).
As described by Hidinger (1996) and Abi-Said & Amr (2012) species composition may be affected by the disturbance caused by the tourist presence. In this sense, the absence of the Central American agouti in the reserve could be due to the high traffic of people (e.g. tourists, staff, researchers) passing on foot, bicycles or motorbikes along the human-made trail used to set up the camera trap stations. It is possible that the species reduced its activity on the trail to avoid people, which could explain why the camera traps did not record it. The presence of domestic dogs (Canis lupus familiaris) could also be affecting the species distribution and abundance along the trail (Lessa, Corrêa, de Godoy, Cunha & Vieira, 2016). Other possible reasons for its low occurrence could be the habitat degradation that has occurred around the reserve (e.g. large-scale banana and pineapple plantations), as well as illegal hunting of the species for its consumption.
The abundance of jaguars reported in the study area was higher than expected in terms of the area sampled.In this sense, our study recorded five jaguars in only 10,50 km2, while typically jaguar density ranges from 1-2,7 individuals per 100km2, up to 11 individual per 100km2 (Hunter, 2015). The high abundance of jaguars may be related to the availability of prey found in the reserve, which according with some authors (Crawshaw, 1995;Paviolo, de Angelo, Di Blanco & Di Bitetti, 2008) is a key factor in maintaining a healthy jaguar population. Similar results were also obtained in Tortuguero National Park, where jaguar occupancy was correlated with the vast availability of prey (marine turtles; Arroyo-Arce et al., 2014). However, jaguar predation on domestic animals has been reported in the ranches surrounding the reserve (D. Corrales, pers. comm., April 05, 2016). Although these attacks may have been random events, they could also be an indication of low levels of natural prey species. Therefore, further research is needed in order to determine the factors that could better explain the high presence of jaguars in the study area.
Harmsen, Delgado-Jaramillo, Machado & Aular (2011) mentioned that jaguar activity patterns vary across their geographic range in relation to its main prey. Based on this, it is expected that jaguars in the reserve rely more on nocturnal species as prey. Likewise, the nocturnal habit of the ocelot could also be a strategy to overlap with the activity pattern of its main prey (Ludlow & Sunquist, 1987; Moreno, Kays & Samudio, 2006).
Activity patterns obtained for the other three species of mammals agrees with existing literature. Our data categorized the red brocket deer as being both diurnal and nocturnal species, similar results were obtained by Tobler, Carrillo-Percastegui & Powell (2009). The nocturnal behavior of the common opossum and the spotted paca coincided also with previous studies (Harmsen et al., 2011; Michalski & Norris, 2011; Lira-Torres & Briones-Salas, 2012).
The distance covered by the ocelot M014-12 (41km, approximately) is comparable to that described in the literature (Crawshaw, 1995; Jacob, 2002; Booth-Binczik, 2007). As Tortuguero National Park and Pacuare Nature Reserve are not connected through a continuous forest, it is unclear the dispersal route utilized by the felid. However, one can presume that the ocelot travelled through the fragmented landscape (dominated by agriculture fields and human settlements) using the remaining forest patches along the Caribbean coast. This record constitutes the first evidence of wildlife movement between Tortuguero National Park and Pacuare Nature Reserve, and enhances the importance of corridors for the long-term conservation of the species.
Our findings give us a first insight of the mammalian diversity found in the Pacuare Nature Reserve. Further research is recommended in order to have a better knowledge regarding the diversity that host the Reserve, and detect trends in the presence and abundance of species. This information will be important for an effective management of the area.
ACKNOWLEDGEMENTS
We thank the Endangered Wildlife Trust for all their conservation efforts carried out in Pacuare Nature Reserve. The camera trap project was made possible thanks to the cooperation between Pacuare Nature Reserve, Panthera Costa Rica and Coastal Jaguar Conservation. The latter received funding from the Rufford Small Grants Foundation and Idea Wild. We also thank the Unidad de Atención de Conflictos con Felinos (UACFel, organized by the Costa Rican government and Panthera Costa Rica), a specialized unit that help mitigate conflicts over wild felid attacks on farm animals, for providing data regarding the human-wildlife conflict in the surrounding area of the reserve.
REFERENCES
Abella-Gutiérrez, I., & López-Conlon, M. (2008). Fenología reproductiva de una colonia de Garza Agami (Agamia agami, Aves: Ardeidae) en la Reserva Pacuare, Costa Rica. Brenesia, 69, 77-79.
Abi-Said, M., & Amr, Z. (2012). Camera trapping in assessing diversity of mammals in Jabal Moussa Biosphere Reserve, Lebanon. Vertebrate Zoology, 62, 145-152.
Ahumada, J., Hurtado, J., & Lizcano, D. (2013). Monitoring the status and trends of tropical forest terrestrial vertebrate communities from camera trap data: a tool for conservation. Plos One. Available online at http://dx.doi.org/10.1371/journal.pone.0073707.
Ahumada, J., Silva, C., Gajapersad, K., Hallam, C., Hurtado, J., Martin, E., McWillam, A., Mugerwa, B., O’Brien, T., Rovero, F., Sheil, D., Spironello, W., Winarni, N., & Andelman, S. (2011). Community structure and diversity of tropical forest mammals: data from a global camera trap network. Philosophical Transactions of the Royal Society Biological Sciences, 366, 2703-2711.
Altrichter, M., Taber, A., Beck, H., Reyna-Hurtado, R., Lizarraga, L., Keuroghlian, A., & Sanderson. E. (2011). Range-wide declines of a key Neotropical ecosystem architect, the Near Threatened white-lipped peccaryTayassupecari. Oryx, 46, 87-98.
Arévalo, J. E., Méndez, Y., Roberts, M., Alvarado, G., & Vargas, S. (2015). Monitoring species of mammals using track collection by rangers in the Tilarán mountain range, Costa Rica. Cuadernos de Investigación UNED, 7, 249-257.
Arroyo-Arce, S., Guilder, J., & Salom-Pérez, R. (2014). Habitatfeaturesinfluencing jaguar Panthera onca (Carnivora: Felidae) occupancy in TortugueroNational Park, Costa Rica. Revista de Biología Tropical, 62, 1449-1458.
Arroyo-Arce, S., Thomson, I., & Salom-Pérez, R. (2016). Relative abundance and activity patterns of terrestrial mammalian species in Barra del Colorado Wildlife Refuge, Costa Rica. Cuadernos de Investigación UNED.
Beisiegel, B. (2009). First camera trap record of bush dogs in the state of São Paulo, Brazil. Canid News, 12 (5), 1-5.
Booth-Binczik, S. (2007). Report from the field: monitoring ocelot dispersal with satellite telemetry. Endangered Species Update, 24, 110-112.
Chacón-Chaverri, D., & Eckert, K. L. (2007). Leatherback sea turtle nesting at Gandoca Beach in Caribbean Costa Rica: management recommendations from fifteen years of conservation. Chelonian Conservation and Biology, 6, 101-110.
Crawshaw, P. G. (1995). Comparative ecology of ocelot (Felis pardalis) and jaguar (Panthera onca) in a protected subtropical forest in Brazil and Argentina. (Doctoral dissertation). University of Florida, USA.
Cuskelly, M. (2012). Differential nesting in the leatherback sea turtle (Dermochelys coriacea) at Pacuare Nature Reserve, Costa Rica. (Master thesis). Coastal Carolina University, USA.
Eadie, E. C. (2015). Ontogeny of Foraging Competence in Capuchin Monkeys (Cebus capucinus) for Easy versus Difficult to Acquire Fruits: A Test of the Needing to Learn Hypothesis. Plos One. Available online at http://dx.doi.org/10.1371/journal.pone.0138001
García, F., Delgado-Jaramillo, M., Machado, M., & Aular. L. (2012). Preliminary inventory of mammals from Yurubí National Park, Yaracuy, Venezuela with some comments on their natural history. Revista de Biología Tropical, 60, 459-472.
Harmsen, B., Foster, R., Silver, S., Ostro, L., & Doncaster, C. (2011). Jaguar and puma activity patterns in relation to their main prey. Mammalian Biology, 76, 320-324.
Hidinger, L. (1996). Measuring the impacts of ecotourism on animal populations: a case study of Tikal National Park, Guatemala. (Master thesis). Duke University, USA.
Hunter, L. (2015). Wild cats of the world. Bloomsbury Natural History, New York, USA.
Jacob, A. A. (2002). Ecologia e conservação da jaguatirica (Leopardus pardalis) no Parque Estadual Morro do Diabo, Pontal do Paranapanema, SP. (Master thesis). Universidade de Brasília, Brasília, Brazil.
Lessa, I., Corrêa, T., de Godoy, H., Cunha, A., & Vieira, E. (2016). Domestic dogs in protected areas: a threat to Brazilian mammals? Natureza & Conservação. Available online at doi:10.1016/j.ncon.2016.05.001
Lira-Torres, I., & Briones-Salas, M. (2012). Abundancia relativa y patrones de actividad de los mamíferos de los chimalapas, Oaxaca, México. Acta Zoológica Mexicana, 28, 566-585.
Ludlow, M. E., & Sunquist, M. E. (1987). Ecology and behavior of ocelots in Venezuela. National Geographic Research, 3, 447-461.
Maffei, L., Noss, A. J., Cuéllar, E., & Rumiz, D. I. (2005). Ocelot (Felis pardalis) population densities, activity, and ranging behaviour in the dry forests of eastern Bolivia: Data from camera trapping. Journal of Tropical Ecology, 21, 1-6.
Mérida, J. & Cruz, G. (2015). Primer registro del marsupial Metachirus nudicaudatus en Honduras (Reserva Biósfera Río Plátano). Cuadernos de Investigación UNED, 7, 337-339.
Michalski, F., & Norris, D. (2011). Activity pattern of Cuniculus paca (Rodentia: Cuniculidae) in relation to lunar illumination and other abiotic variables in the southern Brazilian Amazon. Zoologia, 28, 701-708.
Monroy-Vilchis, O., Zarco-González, M. M., Rodríguez-Soto, C., Soria-Díaz, L., & Urios, V. (2011). Fototrampeo de mamíferos en la Sierra Nanchititla, México: abundancia relativa y patrón de actividad. Revista de Biología Tropical, 59, 373-383.
Moreno, R. S., Kays, R. W., & Samudio, R. (2006). Competitive release in diets of ocelot (Leopardus pardalis) and puma (Puma concolor) after jaguar (Panthera onca) decline. Journal of Mammalogy, 87, 808-816.
Mortimer, J. A., & Donnelly, M. (2008). Eretmochelys imbricata. The IUCN Red List of Threatened Species. Available online at http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T8005A12881238.en
Navarro, J., & Gómez, A. (2015). Diversidad de mamíferos terrestres en bosques cercanos a cultivos de piña, Cutris de San Carlos, Costa Rica. Cuadernos de Investigación UNED, 7, 59-65.
Paviolo, A., de Angelo, C., Di Blanco, Y., & Di Bitetti, M. (2008). Jaguar population decline in the Upper Paraná Atlantic forest of Argentina and Brazil. Oryx, 42, 554-561.
Rivas, M. L., Fernández, C., & Marco, A. (2015a). Nesting ecology and population trend of leatherback turtles Dermochelys coriacea at Pacuare Nature Reserve, Costa Rica. Oryx, 50, 274-282.
Rivas, M. L., Santidrián, R., Diéguez, J., & Marco. A. (2015b). Leatherback hatchling sea-finding in response to artificial lighting: interaction between wavelenght and monnlight. Journal of Experimental Marine Biology and Ecology, 463, 143-149.
Sarmiento-Ramírez, J. M., Abella-Pérez, E., Phillott, A. D., Sim, J., van West, P., Martín, M. P., Marco, A., & Diéguez-Uribeondo, J. (2014). Global distribution of two fungal pathogens threatening endangered sea turtles. Plos One. Available online at doi:10.1371/journal.pone.0085853
Seminoff, J. A. (2004). Chelonia mydas. The IUCN Red List of Threatened Species. Available online at http://dx.doi.org/10.2305/IUCN.UK.2004.RLTS.T4615A11037468.en
Springer, M. T., Carver, A. D., Nielsen, C. K., Correa, N. J., Ashmore, J. R., & Lee, J. G. (2012). Relative abundance of mammalian species in a central Panamanian rainforest. Revista Latinoamericana de Conservación, 2, 19-26.
Tobler, M. W., Carrillo-Percastegui, S. E., & Powell, G. (2009). Habitat use, activity patterns and use of mineral licks by five species of ungulate in South-Eastern Peru. Journal of Tropical Ecology, 25, 261-270.
Troëng, S., Chacón, D., & Dick, B. (2004). Possible decline in leatherback turtle Dermochelys coriacea nesting along the coast of Caribbean Central America. Oryx, 38, 395-403.
Wallace, B. P., Tiwari, M., & Girondot, M. (2013). Dermochelys coriacea. The IUCN Red List of Threatened Species. Available online at http://dx.doi.org/10.2305/IUCN.UK.2013-2.RLTS.T6494A43526147.en
RESUMEN: Abundancia relativa y patrones de actividad de los mamíferos terrestres en la Reserva Natural Pacuare, Costa Rica. Nuestro estudio representa el primer monitoreo de la población local de mamíferos terrestres. Los datos fueron recolectados con siete cámaras trampa. Entre el periodo abril 2015-marzo 2016, y después de 1 643 noches de muestreo, identificamos 11 especies de mamíferos terrestres distribuidos en siete órdenes y nueve familias. La especie más abundante fue el zorro pelón (Didelphis marsupialis), seguido por el ocelote (Leopardus pardalis). La lista de especies que elaboramos para la reserva es semejante a las de otras áreas protegidas del noreste caribeño de Costa Rica.
Palabras clave: mamíferos; abundancia relativa; patrón de actividad; cámaras trampa; Costa Rica.
Fig. 1. Study area and camera trap distribution in Pacuare Nature Reserve, Costa Rica.

TABLE 1
Relative abundance index for mammalian species recorded by camera traps in Pacuare Nature Reserve, Costa Rica
|
Order |
Family |
Species |
RAI1 |
|
Carnivora |
Felidae |
Panthera onca |
2,01 |
|
|
|
Puma concolor |
0,06 |
|
|
|
Leopardus pardalis |
2,43 |
|
|
Mustelidae |
Eira barbara |
0,18 |
|
|
Procyonidae |
Procyon lotor |
0,61 |
|
Cetartiodactyla |
Cervidae |
Mazama temama |
2,01 |
|
|
Tayassuidae |
Pecari tajacu |
0,30 |
|
Cingulata |
Dasypodidae |
Dasypus novemcinctus |
0,18 |
|
Rodentia |
Cuniculidae |
Cuniculus paca |
1,03 |
|
Didelphimorphia |
Didelphidae |
Didelphis marsupialis |
5,54 |
|
Pilosa |
Myrmecophagidae |
Tamandua mexicana |
0,06 |
|
1. RAI: relative abundance index. |
|||