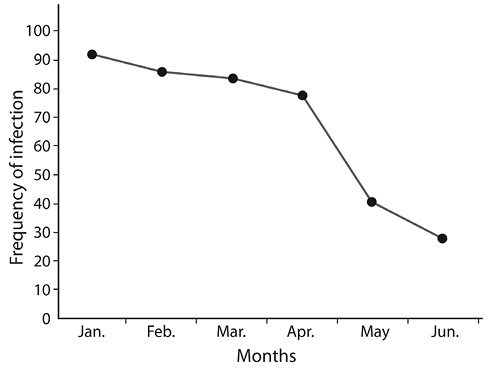
Fig. 1. Distribution of infection by month.
Prevalence of diarrhoea, and associated risk factors,
in children aged 0-5 years, at two hospitals
in Umuahia, Abia, Nigeria
Adanma Florence Nwaoha1, Camelita Chima Ohaeri1 & Ebube Charles Amaechi1, 2
1. Department of Zoology and Environmental Biology, Michael Okpara University of Agriculture, Umudike.
2. Department of Zoology, University of Ilorin, Ilorin, Nigeria; ebubeamechi@yahoo.com
Received 29-VIII-2016 • Corrected 13-X-2016 • Accepted 06-XI-2016
ABSTRACT: Diarrhoea is the second leading cause of infectious morbidity and mortality in children under five years of age. This study aimed at identifying the most common parasites and potential risk factors for diarrhoea among children 0-5 years attending Abia State Specialist hospital and Federal Medical Centre, Umuahia, in south eastern Nigeria. We used 400 faecal samples from children with diarrhoea –and 200 without– in combination with hospital-based case control and a questionnaire Stool samples were processed with direct normal saline and formal-ether sedimentation method for parasitological studies. More males than females were infected in nearly all age groups in both diarrhoeal and control groups (X2=23.04, df=1, P<0.05: X2=11.52, df=1, P<0.05 respectively). Amachara had more infections (X2=0.15, df=1, P< 0.05). January had the highest rate of infection (22.5%). Main clinical features were watery depositions over 3 times a day, diarrhoea lasting for days, fever, vomiting, and dehydration. Mothers learned about the problem through health workers, television and in medical centers. Risk correlated with mother’s education, occupation, latrine type, waste water disposal, hand washing, kitchen cleaning; sources and storage of water; and bottle milk (P< 0.05).Ignorance greatly contributed to the spread of parasitic disease in the area: the government should improve education and other strategies to alleviate the spread of the disease.
Key words: Diarrhoea, Risk factor, Hygiene, Umuahia, Nigeria.
Diarrhoea remains a major public health problem especially in developing countries where it is a leading cause of childhood morbidity and mortality (Mengistie, Berhane & Worku, 2013). An estimated one billion episodes and 2.5 million deaths occur each year among children under five years of age. About 80% of deaths due to diarrhoea occur in the first years of life (Kosek, Bern & Guerrant, 2003). Globally, the majority of diarrhoeal associated deaths occur in Africa and South Asia (UNICEF/WHO, 2009).An estimated half of deaths from diarrhoea among young children occur in Africa, where diarrhoea is the largest cause of death among children under five years of age and also a major cause of childhood illness (Black, Morris & Bryce, 2003; Fisher Walker et al., 2012).
Diarrhoea is a health problem that is connected to water and sanitation, therefore can be both ‘water borne’ and ‘water washed’. Transmission of agents that cause diarrhoea are usually by the faecal oral route, which include the ingestion of faecal contaminated water or food, person to person contact and direct contact with infected faeces (Andu et al., 2002). Epidemiological studies of diarrhoea have been reported from several African countries (Presteryl et al., 2003; Rao et al., 2003). Cases of diarrhoea have long term complications like malnutrition, growth retardation and immune impairment. Studies have shown that to effectively prevent diarrhoea associated illness and death, evaluating the local risk factors associated with diarrhoea should be identified first in that community. Local epidemiology of diarrhoea and associated risk factors in most rural areas of Umuahia is scanty. This study aimed at identifying local risk factors for diarrhoeal illness among children aged 0-5 years attending Abia State Specialist hospital and Diagnostic centre Amachara and Federal Medical Centre Umuahia. It is hoped that the result obtained from this study will be used for the formation and implementation of a wholistic intervention program at controlling the infections.
MATERIALS AND METHODS
Study Area: The study was carried out in two hospitals in Umuahia namely, Federal Medical Centre and Abia State Specialist Hospital and Diagnostic Centre, Amachara (annex), which is located at Amachara Umuokpara, Umuahia South Local Government Area respectively. Umuahia is the capital of Abia State, South East Nigeria. The State is endowed with abundant mineral and agricultural resourses, characterized by a long dry season (November-March) and a longer rainy season (April-October). Umuahia covers a land mass of 245km2 with latitude and longitude of 5o33’20”N and 7o28’52”E respectively, having a population of 359 230 (Ijioma, 1993). Umuahia comprises of two local government areas, Umuahia North and Umuahia south. Federal Medical Center is a tertiary hospital, located at the heart of the town in Umuahia North with a tripartite mandate of service delivery while Abia Specialist Amachara is located in Amachara in Umuahia South local government area. Amachara is a bustlingvillage filled with diverse residents from all works of life.The study population involved 600 infants and young children less than 5 years of age, who presented at the paediatric wards of the Abia State Specialist Hospital and Diagnostic Centre Amachara and paediatric wards of Federal Medical Centre Umuahia, after approval by the ethical committee of the hospitals. The study lasted for 6 months. For the purpose of this study 400 of the subjects were patients attending the hospital as a result of their illness (Diarrhoea) while 200 subjects were children attending the paediatric out-patient clinic, used as control. Since the children were too young at this age, to be interviewed, their parents were interviewed to identify the risk factors for diarrhoea and the contents of the consent form was spelt out to them. To ensure that cases selected for the study represented a homogeneous entity, a strict definition of diarrhoea was established.A case was defined as a child of the first five years of age having three or more loose liquid or watery stools or at least one bloody loose stool within 24 hours.Persistent diarrhoea will be defined as diarrhoea that began acutely and lasted for at least 14 days.Control defined as non-diarrhoea patients, 0-5 years, attending the pediatric out- patient clinic.
RESULTS
Characteristics of the study sample: A total of 600 stool samples of children under five years of age, were investigated in the study after meeting the inclusion criteria. Interviews based on questionnaire were conducted with their mothers. For the group of infants suffering from diarrhoea, 400 stool samples were collected while 200 were collected from non-diarrhoea children which served as control and analyzed in the laboratory to identify parasites causing diarrhoea in them and its associated risk factors (Table 1).
Table 1
Sex and Age distribution of children infected
|
Age Group |
Frequency |
Total |
|
|
Male |
Female |
||
|
Under 6 months |
30 |
12 |
42 |
|
6-11 months |
90 |
48 |
138 |
|
12-23 |
96 |
62 |
158 |
|
24-36 |
30 |
30 |
60 |
|
37-59 |
2 |
0 |
2 |
|
Total |
248 |
152 |
400 |
Distribution of infected children by month: Data on distribution of diarrhoea by month is shown in Figure 1. Among 400 children with diarrhoea, 90 (22.5%) were recruited in January 2015; 21% in February, 20.5%, 19.0% and 10.0% in March, April, and May respectively, there were only 7% recruited in June, because the number of 400 infected children was reached on 25th June 2015, and there was a significant correlation between diarrhoea and month. Seasonality was observed in the distribution of diarrhoea infection with more parasites recovered in the dry season than in wet season.
In this study, distribution of diarrhoea cases by month showed a decreased number of cases among those recruited in the months of April , May and June and as compared to other months. This transitional period between rainy season and dry season may result in the lower number of children with diarrhoea. It was observed that the incidence and severity of this disease may vary depending on the location and period of time (Tumwine et al., 2002).
Distribution of infection by hospitals: With regard to geographical distribution of diarrhoea infection, of the (2) two hospitals used, Abia State Specialist and Diagnostic Centre Amachara and Federal Medical Centre was reported having children infected.
Detailed number and percentage (%) of infection occurring in the above mentioned hospitals are shown in tables from 2 to 6 below, and Abia Specialist Amachara recorded the highest result of 30.0% infected children and 7.0% for control while Federal Medical Centre recorded 24.5 % infected children and 6.0% for control.
DISCUSSION
It is widely recognized that infectious diarrhoea is a major world’s leading cause of morbidity and mortality, resulting in about two million deaths per year, especially children in developing countries (Fewtrell et al., 2005; WHO, 2010). Nigeria is a low income country, mostly Abia state south eastern part where diarrhoea is the second leading cause of deaths among children less than five years of age. Low socio economic status, economic development has been poor due to collapse of infrastructure, environmental degradation; limited education, poor environmental sanitation and low hygienic-practices, all of which pose as a serious threat to people’s health especially children’s health.
Risk factors for diarrhoea vary with the child’s age, the pathogens involved and the local environment. This study is an addition to few studies that have been conducted so far in Abia State. So far, no similar research, identifying the most common causes of diarrhoeaand risk factors associated with diarrhoea among children less than five, has been conducted within these two chosen hospitals.
In this study, high cases were recorded in the month of January 22.5%, which showed that dry weather with high humidity is a good condition for parasites to grow, since the wet season lasted from April to October while the dry season lasted from November to March, that easily led to contamination of food, as many people due to low income cannot afford a refrigerator for food storage. Moreover, unhygienic environmental conditions with presence of cockroaches and flies also increase the rise of diarrhoea as these synanthropic flies can be important in the transmission of diarrhoeal disease causing microorganisms in agreement with the report of Alexander et al., 2013) that diarrhoeal case peaks in both seasons in Botswana, with case 20% more frequent in the dry season than the wet season.In this study, more infection was recorded in Abia State Specialist Hospital & Diagnostic Centre Amachara and than in Federal Medical Centre Umuahia, this might be because of the state of the environment that may have some factors that favours parasites multiplication. Umuahia as a State capital is cleaner and has more educated people that had a very good knowledge of the risk factors of diarrhoea than in Amachara, which invariably might have led to the result outcome of Amachara having more cases. This might be explained using the work of Yassin (2002), which states that in rural areas, due to low income, inadequate water source and unhygienic environment, people suffer more to infectious diseases. This study also revealed that infection was seen in unequal frequencies from children with diarrhoea and healthy control in both hospitals. This observation is in agreement with the work of Parasharet al. (2006) that reported a difference in the frequency of infection in diarrhoea children and healthy control.According to Thielman and Guerrant (2004), the rates of diarrhoea were highest for children 6-11 months of age, remained at a high level among 1-year-old children and decreased when children got older. It was observed in this study that cases of diarrhoea were mostly found among children less than 24 months of age and number of cases decreased in older children.
A decrease in number of cases among older children might be due to fact that the immune system of older children get stronger in resistance against agents of diarrhoea as can be seen in the work of (Gascon et al., 2000), symptoms of a diarrhoea in older children may also be lighter compared to younger children, because they could easily be given over-the-counter-drugs or sent to chemist for treatment. The reason for sex difference regarding rate of diarrhoea is far from clear. This agrees with report of (Ogunlesi et al., 2006; Ogbu et al., 2008; Ohaeri & Odukaesieme, 2011). The Result of the study revealed that number of males were higher than in females, However, showed association (p<0.05). This is consistent with results of other researchers Anasari (2012). In children under 5 years of age in Kathmandu Nepal, Ishiyama et al. (2001), reported higher prevalence in males than females. A biological explanation may be related to the fact that boys during infancy have to build a larger muscle mass than girls. Consequently, boys might have increased demands for micronutrients and are therefore more at risk of a negative balance, including lack of vitamin A and zinc (Jinadu et al., 1991). This vulnerability might increase the risk of diarrhoea and place the boys as the weaker sex regarding infections. Among older children, because boys are more active than girls, boys tend to move around and touch objects in the surrounding ground, whereas girls might tend to stay close to their mothers and play with more hygienic toys.In this study, some factors were found independently associated with the risk of diarrhoea, including, socio-economic factors that related on the child having siblings; due to low public awareness of birth control, many families have many children and some family prefer a son to a daughter in order to maintain family line; as a result of that old thinking, child bearing will continue until the family has a male child.
Categorization of children into those having siblings and children who do not have, it was found that the risk of having diarrhoea among children who have one or more siblings was higher than those who are the only child, (p<0.05) showing significant association between birth order (child having siblings) and diarrhoea and this is different from the results of Gascon et al. (2000) that indicated in his study that there was no significant association between birth order and diarrhoea. Moreover, when the children are many, there may be lack of parental care and unhygienic environmental situation which leads to a higher risk of diarrhoea among children with siblings than among those who are the only child.
Many researchers (Curtis et al., 2000; Amaechi et al., 2013) found that unhygienic latrines and sharing latrines increased the risk of diarrhoea. In some homes, newspaper, old notebook papers are used or other low quality toilet papers are used for cleaning after going to toilet. Those papers cannot be flushed with water but they are usually kept in a bucket placed in the toilet. In this situation, cleaning the latrines less than 3 times per week was considered irregular. Also, the number of 5 people was used as cut-off point to categorize the number of people sharing a latrine into 2 values: more than 5 and 5 downwards. Obviously, more people sharing a latrine reduce the hygienic level of the latrine. The prevalence of diarrhoea from this study was low when compared to other developing countries like Chile and Brazil as was observed by Househam et al. (1998), as a result of sanitary condition and methods of faecel disposal. Improper disposal of child’s stool, using stool as fertilizer and irregular latrine cleaning and sharing latrine among many people are not associated with risk of diarrhoea (p>0.05) and are comparable with the results indicated in a large number of studies so far, like the work of Curtis (2000).
In this study, the risk of diarrhoea among children whose mothers had the two poor hand related practices and children that don’t wash their hands before eating are higher than that among those whose mothers paid attention to washing their hands after going to toilet and before feeding children respectively and also hand washing by mothers with soap and water.
Some researchers have clearly shown in their work, that hand washing in a proper way can reduce the occurrence of diarrhoea as can be seen in the work of Tumwine et al. (2002). Some mothers are weaker compared with fathers, and their tiredness might be a reason for not paying attention to hand washing. In addition, low level of education as well as poor knowledge of diarrhoea also explain why mothers do not realize that their sanitation and food hygiene-related-practices are very important to children’s health.
Irregular kitchen cleaning facilitates the development of diarrhoea transmitting vectors such as flies and cockroaches. In addition, when kitchens are built close to livestock stables, it might cause an unhygienic situation of kitchen. As seen in this study, number of those that practice everyday kitchen cleaning showed a significance association with diarrhoea (p<0.05), but hand washing by mother’s after helping children defecate also showed association with diarrhoea (p<0.05).
From this study, unsafe and safe methods of storing food were found not significant to increase the risk of diarrhoea (p>0.05), this is in contrast with the work of Jinaduet al. (1991), and buying food for children from street vendors showed no significant with diarrhoea. It can be compared with the work of Wilson (2005).
Sources of getting water, storage method and infrequent cleaning / emptying of storage container before refilling it with fresh water were found significant to increase the risk of diarrhoea. This can be compared with the work of Jensen et al. (2002) which found that transmission of diarrhoea occurs easily when in house water storage facilities and water sources are contaminated, which is in agreement with the work of Fewtrell et al. (2005).
In terms of breastfeeding status of the child, it was found in this study that the number of diarrhoea cases in children not exclusively breastfed and those mixed were very high when compared to those not breastfed at all implying that the importance of breastfeeding cannot be over emphasized. Complete exclusive breastfeeding of a baby gives adequate protection against various gastro intestinal diseases.In this study it was seen that those not exclusively breastfed, those not breastfed at all and those that practiced mixed method were found significant to the risk of diarrhoea (P<0.05) when compared with those exclusively breastfed.
In conclusion, childhood diarrhea remains an important heath problem in the study areas. Interventions targeted at improved sanitation, hygiene and better child spacing will go a long way in alleviating the problem.
REFERENCES
Alexander, K. A., Carzolio, M., Goodin, D., & Vance, E. (2013). Climate change is likely to worsen the public health threat of diarrhoeal disease in Botswana. International Journal of Environmental Research and Public Health, 10 (4), 1202-1230.
Amaechi, E. C., Ohaeri, C. C., & Ukpai, O. M. (2013). Prevalence of helminthias is among school children in some rural communities of Abia State, Nigeria. Animal Research International, 10(3), 1817-1825.
Anasari, S., Bahadur, S., Parajuli, K., & Paudyal, R. P. A. (2012). Pattern of acute parasitic diarrhoea in children under five years of age in Kathmandu, Nepal. Open Journal of Medical Microbiology, 2, 95-100.
Andu, R., Omilabu, S. A., Peenze, I., & Steele, D. (2002). Viral diarrhoea in young children in two districts of Africa. Central African Journal for Medicine, 48, 59-63.
Black, R. E., Morris, S. S., & Bryce, J. (2003). Where and why are 10 million children dying every year? Lancet, 361, 2226-2234.
Curtis, V., Cairncross, S., & Yonli, R. (2000). Review: Domestic hygiene and diarrhoea-pinpointing the problem. Tropical Medical International Health, 5 (1), 26–30.
Fewtrell, L., Kaufmann, R. B., Kay, D., Enanoria, W., Haller, L., & Colford J. M, Jr. (2005) Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries:a systematic review and meta-analysis. Lancet Infection Disease, 5 (1), 42-52.
Fisher Walker, L. C., Perin, J., Aryee, J. M., Boschi-Pinto, C., & Black, R. E. (2012). Diarrhea incidence in low and middle income countries in 1990 and 2010: A systematic review. BMC Public Health, 12.
Gascon, J., Vargas, M., Schellenberg, D., Urassa, H., Casals, C., Kahigva, E., Aponte, J. J., Mshinda, H., & Vila, J. (2000). Diarrhoea in Children under 5 Years of Age from Ifakara, Tanzania. Journal of Clinical Microbiology, 38 (12), 4459-4462.
Househam, K. C., Mann, M. D., & Bowie, M. D. (1988). Enteropathogens associated with acute infantile diarrhoea in Cape Town. South Africa Medical Journal, 73, 83-87.
Ijioma, M. A. (1993). Abia State Survey. In: Nigeria, Giant in the tropics, Udo, R. K., & Mamman, A. B. (Eds.) Vol. 2, Gabumo Publishing Co., Lagos, pp 760-762.
Ishiyama, S., Ono, K., & Rai, S. K. (2001). Study of enteropathogen and its predisposing factors in suburban public school children in Kathmandu, Nepal. Nepal Medical College Journal, 3, 5-9.
Jinadu, M. K, Olusi, S. O., Agun, J. I., & Fabiyi, A. K. (1991). Childhood diarrhoea in rural Nigeria. Studies on prevalence, mortality and socio-environmental factors. Journal of Diarrhoeal Disease Research, 9(4), 323-327.
Jensen, P. K, Ensink, J. H., Jayasinghe, G., van der Hoek, W., Cairncross, S., & Dalsgaard, A. (2002). Domestic transmission routes of pathogens: the problem of in-house contamination of drinking water during storage in developing countries. Tropical Medication International Health, 7(7), 604-609.
Kosek, M., Bern, C., & Guerrant, R.L. (2003).The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organization, 81 (3), 197-204.
Mengistie, B., Berhane, Y., & Worku, A. (2013). Prevalence of diarrhea and associated risk factors among children under five years of age in eastern Ethiopia: A cross sectional study. Open Journal of Preventive Medicine, 3, 446-453.
Ogbu, O., Aguumadu, N., Uneke, C. J. & Amadi, E. S. (2008). Aetiology of acute infantile diarrhoea in South Eastern Nigeria: An assessment of microbiological and antibiotic sensitivity profile. The Internet Journal of Third World Medicine, 7(1), 2.
Ogunlesi, T., Okeniyi, J., Oseni, S., Oyelami O., Njokanma, F., & Dedeke, O. (2006). Parasitic etiology of childhood diarrhoea. Indian Journal of Pediatric, 73, 1081-1084.
Ohaeri, C. C., & Odukasieme, O. P. (2011). Survey of geohelminthiasis among primary school children in Umuahia metropolis of Abia State, Nigeria. International Journal of Environmental Health and Human Development, 11(1), 41-45.
Parasher, U. D., Gibson, C. J., Bresee, J. S., & Glass, R. I. (2006).Rotavirus and severe childhood diarrhoea. Emerging Infectious Disease, 12(2), 304-306.
Presterl, E., Zwick, R. H., Reichmann, S., Aichelburg, A., Winkler, S., Kremsner, P., & Granigner, W. (2003). Frequency and virulence properties of diarrhea genic Escherichia coli in children with diarrhoea in Gabon. American Journal of Tropical Medical hygiene, 69, 406-410.
Rao, M. R., Abu-Elyazeed, R., Salvarino, S. J., Naficy, A. B., Wierzba, T. F., Abdel-Messih, I., Shaheen, H., Frenck, R. W., Svennerholm, A. M., & Clemens, J. D. (2003). High disease burden of diarrhoea due to enterotoxigenic Escherichia coli among rural Egyptian infants and young children. Journal of Clinical Microbiology, 41, 4862-4864.
Thielman, N. M., & Guerrant, R. L. (2004). Acute Infectious Diarrhoea. The England Journal of Medicine, 350(1), 38-47.
Tumwine, J. K, Thompson, J. Katua-Katua, M., Mujwajuzi, M., Johnstone, N., & Porras, I. (2002). Diarrhoea and effects of different water sources, sanitation and hygiene behaviour in East Africa. Tropical Medical and International Health, 7(9), 750-756.
WHO. (2010). WHO recommendations on the management of diarrhoea and pneumonia in HIV-infected infants and children: integrated management of childhood illness. World Health Organization.
Wilson, M. E. (2005). “Diarrhoea in non-travelers: risk and etiology”. Clinical Infection Disease, 41 (8), 541-546.
Yassin, K. (2000). Morbidity and risk factors of diarrhoeal disease among under-five children in rural upper Egypt. Journal of Tropical Pediatrics, 46 (5), 282-287.
RESUMEN: Prevalencia de diarrea y factores de riesgo asociados, en niños de 0-5 años de edad, en dos hospitales de Umuahia, Abia, Nigeria. La diarrea es la segunda causa principal de morbilidad y mortalidad infecciosa en niños menores de cinco años de edad. El objetivo de este estudio fue identificar los parásitos más comunes y los posibles factores de riesgo para la diarrea entre niños de 0 a 5 años que acuden al hospital especializado del estado de Abia y al centro médico federal Umuahia, al sureste de Nigeria. Se utilizaron 400 muestras fecales de niños con diarrea y 200 sin ella, en combinación con el control de casos hospitalarios y un cuestionario. Las muestras de heces se procesaron con solución salina normal directa y método de sedimentación formal-éter para estudios parasitológicos. En los grupos de diarrea y control se infectaron más hombres que mujeres en casi todos los grupos de edad (X2 = 23.04, df = 1, P <0.05: X2 = 11.52, df = 1, P <0.05 respectivamente). Amachara tuvo más infecciones (X2 = 0,15, df = 1, P <0,05). Enero tuvo la mayor tasa de infección (22,5%). Las principales características clínicas fueron deposiciones acuosas más de 3 veces al día, diarrea durante días, fiebre, vómitos y deshidratación. Las madres aprendieron sobre el problema a través de los trabajadores de la salud, la televisión y en los centros médicos. El riesgo está correlacionado con la educación de la madre, ocupación, tipo de letrina, eliminación de aguas residuales, lavado de manos, limpieza de la cocina; fuentes y almacenamiento de agua y la leche en botella (P <0.05). La ingesta contribuyó en gran medida a la propagación de enfermedades parasitarias en el área: el gobierno debe mejorar la educación y otras estrategias para aliviar la propagación de la enfermedad.
Palabras clave: Diarrea, factores de riesgo, higiene, Umuahia, Nigeria.

Fig. 1. Distribution of infection by month.
Table 2
Socio-economic factors as risk factors associated with diarrhoea
|
Potential risk factors |
Cases (%) n= 400 |
Controls (%) n = 200 |
X2 |
P-value |
|
Knowledge/levels of mother’s education |
||||
|
Primary |
59.5 |
76.0 |
18.96 |
0 |
|
Secondary |
23.5 |
18.0 |
25.88 |
0 |
|
University |
17.0 |
6.0 |
39.2 |
0 |
|
Marital status |
||||
|
Single |
1.0 |
2.0 |
0 |
1 |
|
Married |
97.8 |
98 |
64.78 |
0 |
|
Divorced |
75.0 |
0 |
3 |
0.08 |
|
Separated |
0.5 |
0 |
2 |
0.16 |
|
Mother’s age under 26 |
45.75 |
40.5 |
39.41 |
0 |
|
Mother’s occupation |
||||
|
Farmer |
57.3 |
77.0 |
14.69 |
0 |
|
Employed |
20.3 |
16.5 |
20.21 |
0 |
|
Self employed |
22.5 |
6.5 |
57.56 |
0 |
|
Father’s occupation |
||||
|
Farmer |
32.8 |
59.5 |
0.58 |
0.45 |
|
Employed |
25.3 |
19.5 |
27.46 |
0 |
|
Self employed |
39.8 |
19.5 |
27.730 |
|
|
Number of child/siblings |
||||
|
The child having siblings |
54.0 |
69 |
17.19 |
0 |
|
No siblings |
46.0 |
31.0 |
60.5 |
0 |
p<0.05 (Significant).
Table 3
Sanitation factors as risk factor of diarrhoea
|
Potential risk factors |
Cases n = 400 (%) |
Controls n = 200 (%) |
X2 |
P-value |
|
Types of latrines used |
||||
|
Old- type latrines |
49.3 |
65 |
13.73 |
0 |
|
Modern toilet |
50.8 |
35 |
64.8 |
0 |
|
Latrine cleaning |
||||
|
1-2 times per week |
15.3 |
36 |
0.91 |
0.34 |
|
Everyday |
34.0 |
30 |
29.47 |
0 |
|
Every time it is spoiled |
50.8 |
34 |
67.25 |
0 |
|
Latrine sharing (≥5 people) |
6.5 |
14 |
0.074 |
0.79 |
|
Disposal of child’s stool |
||||
|
Throw away in open surrounding |
1.4 |
5 |
1.67 |
0.2 |
|
Buried |
2.8 |
3.5 |
0.89 |
0.35 |
|
Put in latrine |
87.3 |
91 |
52.52 |
0 |
|
Disposal of waste water |
||||
|
Pour away in open surrounding |
28.3 |
15.5 |
46.69 |
0 |
|
Sewage system |
71.8 |
84.5 |
30.54 |
0 |
|
Using stool as fertilizer |
1.8 |
6.5 |
1.8 |
0.18 |
Table 4
Hygiene related risk factors of diarrhoea
|
Table 4 (Continued) |
||||
|
Potential risk factors |
n = 400 cases (%) |
n = 200 controls (%) |
X2 |
P-value |
|
Potential risk factors |
n = 400 cases (%) |
n = 200 controls (%) |
X2 |
P-value |
|
No hand washing for child before eating |
37 |
47.5 |
11.56 |
0 |
|
Hand washing by mothers after toilet |
||||
|
Sometimes |
5.5 |
26 |
12.16 |
0 |
|
Usually |
94.5 |
74 |
66.6 |
0 |
|
Hand washing by mothers after helping children defecate |
||||
|
Sometimes |
26.5 |
45.5 |
1.14 |
0.2 |
|
Usually |
73.5 |
54.5 |
84.93 |
0 |
|
Hand washing by mothers before feeding children |
||||
|
Never |
1.8 |
12 |
9.32 |
0 |
|
Sometimes |
42 |
51 |
15.59 |
0 |
|
Usually |
56.3 |
36.5 |
77.53 |
0 |
|
Hand washing by mothers before preparing food for children |
||||
|
Never |
3.5 |
15.5 |
6.42 |
0.01 |
|
Sometimes |
61.5 |
52.5 |
56.64 |
0 |
|
Usually |
35 |
32 |
28.31 |
0 |
|
Hand washing by mothers with |
|
|
|
|
|
Water only |
58.8 |
80.5 |
13.83 |
0 |
|
Water and soap |
41.3 |
19.5 |
77.82 |
0 |
|
Methods of storing food for later use |
||||
|
Unsafe method |
22.5 |
51.5 |
0.88 |
0.35 |
|
Not storing food for later use |
51.5 |
29.5 |
81.54 |
0 |
|
Storing food in refrigerator |
26 |
19 |
30.68 |
0 |
|
Storing food in larder |
11.8 |
35.5 |
4.88 |
0.03 |
|
Not heating food before reuse |
1.3 |
0.5 |
2.67 |
0.1 |
|
Buying food for children from street vendors |
14.5 |
21.5 |
2.22 |
0.14 |
|
Kitchen cleaning |
|
|
|
|
|
Not regularly |
18.8 |
56.5 |
7.68 |
0 |
|
Every day |
81.3 |
43.5 |
137.49 |
0 |
|
2-3 times per week |
17.5 |
35 |
0 |
1 |
|
Every week. |
1.3 |
22.5 |
30.08 |
0 |
|
Flies in the kitchen |
63 |
58.8 |
49.39 |
0 |
|
Animal entering the kitchen |
30.8 |
35 |
14.54 |
0 |
|
keeping animal in the kitchen overnight |
4.75 |
4 |
4.48 |
0.03 |
Table 5
Water related factors as risk factor of diarrhoea
|
Potential risk factors |
Cases n = 400 (%) |
Controls n = 200 (%) |
X2 |
P-value |
|
Getting water from |
||||
|
Unsafe water |
82.5 |
88.5 |
46.17 |
0 |
|
Running water / borehole |
17.5 |
11.5 |
23.75 |
0 |
|
Storage method |
||||
|
Storage container without lid |
16 |
13 |
16.04 |
0 |
|
Storage container with lid |
84 |
87 |
51.46 |
0 |
|
Infrequent cleaning / emptying of storage container before refilling it with fresh water |
14.3 |
40 |
4.17 |
0.04 |
Table 6
Breastfeeding status of the child as risk factor of diarrhoea
|
Potential risk factors |
Cases n = 400 (%) |
Controls n = 200 (%) |
X2 |
P-value |
|
Breastfeeding status of child in the 1st 6 months of life |
||||
|
Not exclusive |
85.8 |
58 |
65.72 |
0 |
|
Exclusive |
14.3 |
19.5 |
3.38 |
0.07 |
|
Mixed |
85 |
79 |
66.51 |
0 |
|
Not at all |
0.75 |
1.5 |
0 |
1 |
p<0.05 (Significant).